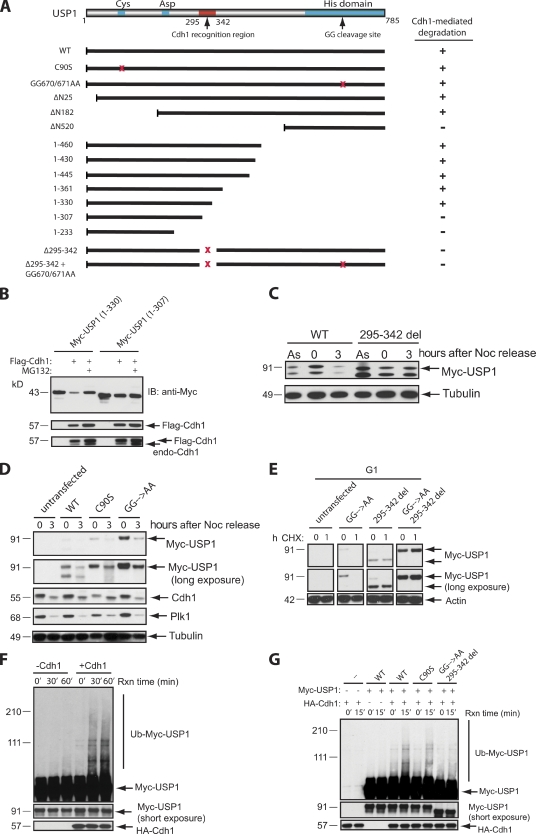
Figure 3.
Identification of a USP1 mutant that cannot be degraded via APC/CCdh1. (A) A schematic diagram of critical USP1 domains and Myc-USP1 expression constructs generated for testing of their stability when coexpressed with FLAG-Cdh1. +, degradation of Myc-USP1; −, stabilization of Myc-USP1; GG, diglycine residue for the USP1 autocleavage site; red x’s, deletion or point mutations of the region. (B) U2OS cells were transiently transfected with Myc-USP1 and/or Flag-Cdh1 constructs in the presence or absence of MG132 (3 h). IB, immunoblot. (C) U2OS cells were transfected with Myc-USP1 wild type (WT) or the 295–342 deletion (295–342 del) mutant and synchronized in M phase with nocodazole (Noc) for 16 h (0 h) and/or released into G1 (3 h after nocodazole release). As, asynchronous. (D) U2OS cells were transfected with Myc-USP1 wild type, C90S, or GG670/671AA and synchronized in M phase or G1 as in C. (E) U2OS cells were transfected with Myc-USP1 wild type, GG670/671AA, 295–342 deletion, or GG670/671AA plus the 295–342 deletion mutant, synchronized, released into G1 (3 h after nocodazole release), and treated with cycloheximide (CHX) for 1 h (1) or not (0). (F) In vitro ubiquitination assay of Myc-USP1 wild type in the presence or absence of HA-Cdh1. Reactions were performed as described in the Materials and methods section. (G) In vitro ubiquitination assay of Myc-USP1 wild type, C90S, and GG670/671AA plus the 295–342 deletion mutant in the presence or absence of HA-Cdh1. Rxn, reaction.