Abstract
Gardnerella vaginalis is a Gram-variable coccobacillus found in the lower genital tract, particularly of women. Very large numbers are found in the vagina in bacterial vaginosis. The pathogenicity of G. vaginalis was studied using fallopian tubes and bovine oviducts in organ culture. Whole organisms, whether piliated or not, from broth cultures caused the cilia on ciliated cells in the mucosa of either human or bovine oviducts to stop beating within 3 days or less. Cilia on control tissues kept beating for at least 5 days. Organism-free filtrates from broth cultures, whether frozen and thawed or heat-treated, caused the same effect, indicating the existence of a soluble toxin. Histological sections revealed little damage, but scanning electron microscopy showed damage to the mucosal surface with some loss of ciliated cells. The toxin is not human tissue specific and, therefore, unlikely to be the same as the cytotoxin with haemolytic properties described by others. The toxin could play a part in the development of salpingitis if G. vaginalis organisms gained access to the upper tract in large numbers.
Keywords: electron microscopy, Gardnerella vaginalis, oviduct organ cultures, pathogenicity
Gardnerella vaginalis is a Gram-variable coccobacillus. It is found in small numbers in the vagina of a large proportion of healthy women, pregnant or not, and is detected in 1000-fold or greater numbers in bacterial vaginosis (BV), together with a multitude of other bacterial species, the individual organisms of which have also increased greatly in number (Rosenstein et al. 1996). Although Gardner & Dukes (1955); considered G. vaginalis (at that time termed Haemophilus vaginalis) to be the cause of ‘non-specific vaginitis’, now termed BV, its exact role is not clear. However, it seems reasonable to suppose that a very large increase in the number of G. vaginalis organisms in the vagina could increase the chance of their access to the upper genital tract and to possible involvement in pelvic inflammatory disease (PID). With this in mind, we have examined the effect of G. vaginalis on human and bovine oviduct organ cultures, in the same way that the effect of other genital-tract micro-organisms, namely Neisseria gonorrhoeae (Johnson et al. 1977; McGee et al. 1990) and Mobiluncus species (Taylor-Robinson et al. 1993), has been investigated. We also compared the effect of piliated and non-piliated strains of G. vaginalis as piliated and non-piliated strains of gonococci behave differently from each other (McGee et al. 1981).
Materials and methods
Bacterial strains
Four strains of G. vaginalis (3.1, 47.3, 194 and 762) were used. The source of these has been described earlier (Boustouller et al. 1986, 1987). Strains 194 and 762 had been shown by electron microscopy to be piliated, whereas the other two strains were not (Johnson & Davies 1984; Boustouller et al. 1987).
Growth and storage of strains
All strains were grown on Columbia agar containing 10% (v/v) defibrinated sheep blood (Difco; Surrey) for 48 h at 37 °C in an atmosphere of 5% CO2 in air. Alternatively, G. vaginalis was grown in peptone–starch–dextrose (PSD) broth (Dunkelberg et al.1970) containing 10% heat-inactivated (56 °C for 30 min) horse serum.
All strains were stored at −70 °C either in thioglycollate broth containing 10% (v/v) glycerol and 10% (v/v) defibrinated sheep blood or in skimmed milk.
Inocula for organ cultures
Viable G. vaginalis organisms were grown for 48 h in PSD broth, washed by centrifugation at 400 g and resuspended in minimal essential medium (MEM)-HEPES, unless otherwise stated, at various concentrations up to 108 colony-forming units (cfu) per ml. Bacterial filtrates were prepared by growing G. vaginalis in PSD broth for 48 h; a sample of the broth was then titrated to determine the number of organisms and was checked for purity. The broth was then filtered through a 0.22-μm ‘Acrodisc’ disposable filter (Gelman Sciences) and the filtrate checked for bacterial contamination. A portion of the filtrate was kept at 4 °C until required, while the remainder was either frozen and thawed twice and then refiltered or heated at 56 °C for 30 min and then refiltered. Controls that comprised filtrates of sterile PSD broth were treated identically.
Preparation, maintenance and inoculation of organ cultures
Fallopian tubes were obtained from non-pregnant, postmenopausal women undergoing hysterectomy. Permission to obtain and use them had been granted by the local Ethics Committee. The tubes were transported within 30 min to the laboratory and immersed in Eagle's MEM containing Earle's salts and l-glutamine (Wellcome Research Labs, Beckenham, UK), diluted to single strength with sterile pyrogen-free water and buffered with 0.05 M N-2-hydroxyethylpiperazine-N-2-ethanesulphonic acid (HEPES; Gibco) as follows: 10 ml Eagle's MEM, 85 ml sterile water, 5 ml 0.05 M HEPES and 10 μg/ml ampicillin (Beechams Research Labs, Brockham Park, Surrey, UK) made up to pH 7.4 with sterile 1 N NaOH.
Bovine oviducts were obtained after Caesarean section delivery of viable offspring at the Agricultural Research Council station at Compton (near Newbury, Berkshire, UK), and transported in the medium described earlier (in packed ice) to the laboratory. Ethical permission for removal and use of the oviducts was not required.
The fallopian tubes and bovine oviducts were dissected in a Microflow cabinet, the adventitial tissue removed and the tubes cut longitudinally through the lumen to expose the mucosal surface. These tissues were then cut into small pieces (3- to 4-mm2), and adjacent pieces were placed mucosa uppermost in a 30-mm petri dish containing 2.5 ml of MEM-HEPES buffer. The dishes were numbered consecutively so that pieces in adjacent dishes were from adjacent regions of the organs. The dishes were incubated at 37 °C overnight. After assessment of the initial ciliary activity, as described elsewhere, the medium from each dish was removed and checked for bacterial contamination. Each dish then received 2.5 ml of the inoculum under investigation or appropriate control medium and was incubated at 37 °C.
Subsequent to inoculation, the number of G. vaginalis organisms expressed as colony-forming units (cfu) per ml in the medium of the organ cultures was determined daily by making serial 10-fold dilutions of the medium in sterile PBS, plating 10-μl aliquots on Columbia blood agar and incubating as described previously. Media from organ cultures that had received filtrates were cultured daily to confirm their sterility.
Extent and quantitation of ciliary activity
As many tissue pieces as possible were cut from a tube in order to provide at least two dishes, each containing three pieces of tissue, for each variable tested. This allowed the removal of a piece, if required, for staining or electron microscopical examination without jeopardizing the scoring of ciliary activity. The tissue pieces were examined ‘blind’ in that there was no preknowledge of which tissues had received G. vaginalis organisms or filtrates and those that had not and were acting as controls. The proportion of the periphery of each piece of tissue where cilia were beating was assessed by a single observer (Y.L.B) using an inverted microscope (Olympus) at 100× magnification. Ciliary activity or vigour was assessed subjectively on a scale of 0–10, where 0 is ‘no activity’ and 10 is rapid beating along the whole edge. The mean scores for at least three pieces at different times of observation were expressed as a percentage of the score at the beginning of the experiment. The significance of the differences between scores for G. vaginalis-inoculated and uninoculated organ cultures was determined by Student's two-sided t test (P values given in the Tables). The methods have been described in detail previously (McGee et al. 1976).
Assessment of ciliary and tissue damage
Ciliary activity, determined as described earlier, was monitored daily. Tissue pieces were removed at intervals for examination by light or scanning electron microscopy. For light microscopy, the pieces were fixed overnight in formol sublimate, then embedded in wax and ultrathin sections cut and stained using haematoxylin and eosin. Some sections were stained with Gram-methyl green-pyronin light green (MGPLG) as described by Sowter and McGee (1976). Stained sections were examined at 400× magnification. For scanning electron microscopy, tissues were rinsed once in PBS and then fixed overnight at 4 °C in freshly prepared 3% glutaraldehyde solution in 0.1 M sodium cacodylate buffer, pH 7.3. The tissues were then rinsed three times in 0.1 M cacodylate buffer and postfixed for 1 h in 1% osmium tetroxide buffered with 0.1 M cacodylate buffer. The fixed tissues were dehydrated in graded solutions of ethanol, dried at the critical point of liquid CO2 and mounted on aluminium studs with ‘Electrodrag 915’ (Acheson Colloids Co., Plymouth, UK) before they were coated with a thin layer of gold and examined in a Phillips scanning electron microscope.
Results
Effect of G. vaginalis on Fallopian tube organ cultures
Two strains of G. vaginalis (3.1 and 762), the former non-piliated and the latter piliated, were suspended at a concentration of 1 × 107 cfu per ml in 50% MEM-HEPES/50% PSD broth. The tissue pieces were then incubated aerobically at 37 °C in this medium, and pieces without organisms (controls) were treated likewise. After 24 h, the ciliary activity of tissue pieces incubated with either of the strains of G. vaginalis had effectively ceased. The concentration of viable organisms was 1 × 104 cfu per ml, a decrease of at least 1000-fold, so that there was no evidence of organism multiplication. In contrast, the ciliary activity of control pieces was 100% at this time and diminished slowly thereafter (Table 1; upper section).
Table 1.
Effect of cultures of Gardnerella vaginalis (strains 3.1 and 762) on the ciliary activity of fallopian tube organ cultures and of fluids removed from the latter after 24 h, filtered and some filtrates frozen and thawed before application to fresh organ cultures
| Ciliary activity (%) at indicated time | |||||||
|---|---|---|---|---|---|---|---|
| Inoculum | Dilution | 0 | 12 h | 24 h | 36 h | 2 days | 3 days |
| Strain 3.1 culture | Nil | 100 | 60* | 5*** | 0 | 0 | |
| Strain 762 culture | Nil | 100 | 50** | 0 | 0 | 0 | |
| Control | Nil | 100 | 100 | 100 | 95 | 80 | |
| Strain 3.1 filtrate | Nil | 100 | 70* | 0 | 0 | 0 | |
| Strain 762 filtrate | Nil | 100 | 60* | 10*** | 0 | 0 | |
| Control | Nil | 100 | 95 | 90 | 85 | 80 | |
| Strain 3.1 filtrate | 1:4 | 100 | 80* | 10*** | 0 | 0 | 0 |
| Strain 3.1 filtrate† | 1:4 | 100 | 75* | 15*** | 5*** | 0 | 0 |
| Strain 762 filtrate† | 1:4 | 100 | 60 | 30** | 10*** | 0 | 0 |
| Control | 100 | 100 | 100 | 95 | 90 | 85 | |
Significance of difference compared to control:
P > 0.05
P < 0.05
P < 0.001.
Frozen and thawed twice.
The medium from the organ cultures described earlier that had received strains 3.1 and 762 of G. vaginalis, including organism-free controls, was removed after 24 h and filtered. The filtrates were checked for sterility before being inoculated into dishes of fresh organ culture pieces. The organism-free filtrates from organ cultures containing organisms had a similar inhibitory effect on ciliary activity as shown for organ cultures containing organisms (Table 1; mid-section).
Sterile filtrates from freshly prepared cultures of G. vaginalis and filtrates frozen and thawed twice were added to fallopian tube organ cultures at a dilution of 1:4. Tissue receiving the 3.1 strain filtrate was devoid of ciliary activity after 36 h and the same filtrate that had been frozen and thawed twice was almost equally as effective; the 762 strain filtrate, frozen and thawed twice, had similar activity. In contrast, the cilia of the controls were 85% active after 3 days (Table 1; lower section). After this time, the pH of the medium containing filtrates was 6.0–6.5 and that of the controls 7.0. Thus, tissue pieces were also placed in MEM-HEPES with the pH adjusted to 6.5 and 6.0. After incubation for 24 h, the ciliary activity of these pieces was comparable with that of the control pieces in the medium of pH 7.0 (data not shown in Table 1). There was no indication, therefore, that loss of ciliary activity was because of an altered pH of the medium.
Tissue pieces taken from cultures after 3 days, sectioned and stained with haematoxylin and eosin showed no histological difference between those that had been in contact with G. vaginalis and those that had not.
Effect of G. vaginalis on bovine oviduct organ cultures
Two strains of G. vaginalis (see Materials and Methods) were grown for 48 h, and each was resuspended in MEM-HEPES at a concentration of 1 × 108 cfu per ml in the oviduct organ cultures. After incubation at 37 °C for 24 h, viable organisms were not detected. The effect on ciliary activity of strains 47.3 and 194 is shown in Table 2 (upper section). By 2–3 days, the ciliary activity of the bovine oviduct pieces bathed in medium containing the organisms had ceased or was significantly reduced, whereas the ciliary activity of control tissues was almost unaffected, beating of most cilia continuing for at least 5 days.
Table 2.
Effect of cultures and culture filtrates of Gardnerella vaginalis (strains 47.3 and 194) on the ciliary activity of bovine oviduct organ cultures
| Ciliary activity (%) at indicated time (days) | |||||||
|---|---|---|---|---|---|---|---|
| Inoculum | Dilution | 0 | 1 | 2 | 3 | 4 | 5 |
| Culture of strain 47.3 | Nil | 100 | 25*** | 0 | |||
| Culture of strain 194 | Nil | 100 | 90* | 40** | 10*** | 0 | |
| Control | 100 | 100 | 100 | 95 | 85 | 80 | |
| Filtrate† of strain 47.3 | 100 | 70* | 35** | 0 | 0 | 0 | |
| Filtrate† of strain 194 | 100 | 55** | 15*** | 5*** | 0 | 0 | |
| Control | 100 | 100 | 100 | 95 | 90 | 85 | |
| Filtrate‡ of strain 47.3 (heated)§ | 1:4 | 100 | 80* | 40** | 5*** | 0 | 0 |
| 1:20 | 100 | 95 | 50 | 40** | 10 | 0 | |
| Filtrate‡ of strain 194 (heated)§ | 1:4 | 100 | 60 | 35** | 10*** | 0 | 0 |
| 1:20 | 100 | 100 | 45** | 30** | 15*** | 5 | |
| Control | 100 | 100 | 100 | 90 | 90 | 80 | |
Significance of difference compared to control:
P > 0.05
P < 0.05
P < 0.001.
Organ culture fluid
Primary culture
56 °C for 30 min.
The medium from all the dishes was removed, filtered and checked for sterility before being used to inoculate fresh bovine oviduct cultures (Table 2; mid-section). Tissue pieces receiving a filtrate of the medium that had previously contained G. vaginalis organisms showed no or minimal ciliary activity after incubation for 3 days, whereas the ciliary activity of the controls was almost unaffected.
Tissue (taken after 3 days in the experiment described earlier) that had been subjected to strains 47.3 and 194 of G. vaginalis, sectioned and stained by MGPLG revealed the presence of a few organisms but no evidence of their attachment to the mucosal surface. Histological sections of the inoculated bovine oviduct pieces stained with haematoxylin and eosin did not reveal obvious pathological changes in the cellular structure when compared with control sections. However, scanning electron microscopy showed that in comparison with the healthy appearance of control tissue (Figure 1), there was damage to the ciliated surface, with sloughing of some of the ciliated cells (Figure 2a,b).
Figure 1.
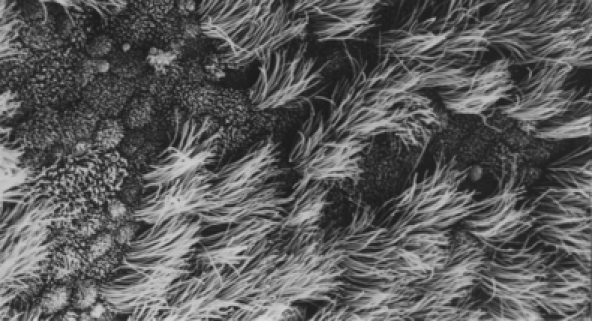
Scanning electron micrograph (× 1772) of bovine oviduct organ culture after contact with filtrate of control broth, diluted 1:4. Tissue fixed after 3 days of incubation. Note healthy mucosal epithelium of ciliated and non-ciliated cells.
Figure 2.

Scanning electron micrograph (× 1772) of bovine oviduct organ cultures showing damage to mucosal epithelium after: (a) Contact with an organism-free filtrate, diluted 1:4, of a culture of Gardnerella vaginalis (strain 47.3). Tissue fixed after 48-h incubation. Note some intact oviduct cilia with rounding (blebbing) of some cells and others detaching. (b) Contact with organism-free filtrate, diluted 1:20, of a culture of G. vaginalis (strain 194). Tissue fixed after 5 days. Ciliated cells and microvilli intact in some areas; in others, ciliated cells detaching.
Cultures of strains 47.3 and 194 of G. vaginalis, grown to a concentration of 1 × 108 cfu per ml, were filtered, and the filtrates were heat-treated and diluted 1:4 and 1:20 in MEM-HEPES before being added to bovine oviduct cultures. As shown in Table 2 (lower section), the filtrates caused a loss of ciliary activity, the rapidity depending on their dilution, filtrates diluted 1:4 causing almost complete loss of activity in 2–3 days, while those diluted 1:20 did so in 4–5 days. These times were considerably shorter than for the control pieces, also bathed in a filtrate of broth and MEM-HEPES, which showed little loss of activity after 5 days. Again, the histological sections showed no difference between the control and inoculated bovine oviducts, whereas scanning electron microscopy revealed that the ciliated surface of the control pieces had a normal appearance but there was damage to the inoculated pieces with sloughing of cells.
Discussion
A drawback of the organ culture system is that it is divorced from the immune system of the host. Despite this, it has provided a valuable tool for examining the pathogenicity of bacterial species found in the genital tract (Taylor-Robinson & Carney 1973; Ward et al. 1974; McGee et al. 1976; Johnson et al. 1977; Taylor-Robinson et al. 1993). In the case of G. vaginalis, we alluded previously (Taylor-Robinson & Hay 1997) to the use of organ cultures but did not provide any detail. In addition to fallopian tubes, bovine oviducts were used because of the comparative ease with which they could be obtained and not specially for examining tissue specificity. However, the results showed that the effect of G. vaginalis was not human tissue specific because damage occurred to both human and bovine oviduct tissues. This is in contrast to the effect of N. gonorrhoeae which, apart from causing damage to chimpanzee oviduct organ cultures (McGee et al. 1990), appears otherwise to be entirely human oviduct tissue specific in its damaging effect (Johnson et al. 1977), correlating with the very narrow host specificity of this bacterium and, therefore, the difficulty experienced in establishing an animal model of gonococcal infection. Piliated gonococci caused more rapid and greater damage than non-piliated organisms possibly because of greater attachment of the former to host tissues. However, the damaging effect of G. vaginalis on bovine and human oviduct tissues occurred irrespective of whether the organisms were piliated or not and was rapid, ciliary activity being greatly reduced or ceasing completely within 2 days, in contrast to persistent activity in controls without organisms. Such cessation occurred following inoculation of organisms in numbers comparable with those found in BV but did not require the persistence of viable organisms. Indeed, viable organisms declined in number rapidly and the damaging effect was seen with organism-free filtrates of broth cultures, or with organ culture medium filtered free of organisms. Little or no damage was seen in the histological sections of the tissues in contact with filtrates or viable organisms; there was no invasion of the mucosal epithelial cells as seen to occur with gonococci (McGee et al. 1981). However, damage was clearly evident in scanning electron micrographs in which there were blebbing and some detachment of whole ciliated cells. It is difficult to distinguish between cessation of ciliary beating and loss of cilia or loss of ciliated cells by naked eye observation of organ cultures; cilia become far less visible once the wave motion stops, giving the impression of loss. Thus, the discrepancy between the histology and naked eye observations is explicable and suggests that much of the loss of ciliary activity seen visually was because of ciliary stasis rather than loss of cilia or ciliated cells, although the latter damage did occur to some extent as indicated by the changes seen on scanning electron microscopy.
The human-specific pore-forming cytolytic exotoxin with haemolytic properties known to be produced by G. vaginalis (Rottini et al. 1990; Cauci et al. 1993) and known to cause damage to vaginal epithelial cells is termed a vaginolysin (Gelber et al. 2008). The substance responsible for ciliary stasis and mucosal damage in the current experiments would seem to be different because of its lack of host tissue specificity. The relationship between the two requires further study, the effect of vaginolysin on bovine oviducts being of interest. The results of the current experiments are another indication of how large numbers of G. vaginalis organisms could contribute to the detachment of epithelial cells from the vaginal mucosa with the formation of ‘clue’ cells (Taylor-Robinson 1984; Cook et al. 1989). Furthermore, the results suggest that access of the organisms to the upper genital tract could contribute to PID (Eschenbach et al. 1988), particularly through disturbance of fallopian tube ciliary activity.
References
- Boustouller YL, Johnson AP, Taylor-Robinson D. Detection of a species-specific antigen of Gardnerella vaginalis by western blot analysis. J. Gen. Microbiol. 1986;132:1969–1973. doi: 10.1099/00221287-132-7-1969. [DOI] [PubMed] [Google Scholar]
- Boustouller YL, Johnson AP, Taylor-Robinson D. Pili on Gardnerella vaginalis studied by electron microscopy. J. Med. Microbiol. 1987;23:327–329. doi: 10.1099/00222615-23-4-327. [DOI] [PubMed] [Google Scholar]
- Cauci S, Monte R, Ropele M, et al. Pore-forming and haemolytic properties of the Gardnerella vaginalis cytolysin. Mol. Microbiol. 1993;9:1143–1155. doi: 10.1111/j.1365-2958.1993.tb01244.x. [DOI] [PubMed] [Google Scholar]
- Cook RL, Reid G, Pond DG, Schmitt CA, Sobel JD. Clue cells in bacterial vaginosis: immunofluorescent identification of the adherent gram-negative bacteria as Gardnerella vaginalis. J. Infect. Dis. 1989;160:490–496. doi: 10.1093/infdis/160.3.490. [DOI] [PubMed] [Google Scholar]
- Dunkelberg WE, Skraggs R, Kellog DS. Method for isolation and identification of Cornybacterium vaginale (Haemophilus vaginalis) Appl. Microbiol. 1970;19:47–52. doi: 10.1128/am.19.1.47-52.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am. J. Obstet. Gynecol. 1988;158:819–828. doi: 10.1016/0002-9378(88)90078-6. [DOI] [PubMed] [Google Scholar]
- Gardner HL, Dukes CD. Haemophilus vaginalis vaginitis. A newly defined specific infection previously classified ‘non-specific’ vaginitis. Am. J. Obstet. Gynecol. 1955;69:962–976. [PubMed] [Google Scholar]
- Gelber SE, Aguilar JL, Lewis KL, Ratner AJ. Functional and phylogenetic characterization of vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J. Bacteriol. 2008;190:3896–3903. doi: 10.1128/JB.01965-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AP, Davies HA. Demonstration by electron microscopy of pili on Gardnerella vaginalis. Br J. Vener. Dis. 1984;60:396–397. doi: 10.1136/sti.60.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AP, Taylor-Robinson D, McGee ZA. Species specificity of attachment and damage to oviduct mucosa by Neisseria gonorrhoeae. Infect. Immun. 1977;18:833–839. doi: 10.1128/iai.18.3.833-839.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee ZA, Johnson AP, Taylor-Robinson D. Human fallopian tubes in organ culture: preparation, maintenance, and quantitation of damage by pathogenic organisms. Infect. Immun. 1976;13:608–618. doi: 10.1128/iai.13.2.608-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee ZA, Johnson AP, Taylor-Robinson D. Pathogenic mechanisms of Neisseria gonorrhoeae: observations of damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J. Infect. Dis. 1981;143:413–422. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- McGee ZA, Gregg CR, Johnson AP, Kalter SS, Taylor-Robinson D. The evolutionary watershed of susceptibility to gonococcal infection. Microb. Pathog. 1990;9:131–139. doi: 10.1016/0882-4010(90)90087-7. [DOI] [PubMed] [Google Scholar]
- Rosenstein IJ, Morgan DJ, Sheehan M, Lamont RF, Taylor-Robinson D. Bacterial vaginosis in pregnancy: distribution of bacterial species in different gram-stain categories of the vaginal flora. J. Med. Microbiol. 1996;45:120–126. doi: 10.1099/00222615-45-2-120. [DOI] [PubMed] [Google Scholar]
- Rottini G, Dobrina A, Forgiarini O, Nardon E, Amirante GA, Patriarca P. Identification and partial characterization of a cytolytic toxin produced by Gardnerella vaginalis. Infect. Immun. 1990;58:3751–3758. doi: 10.1128/iai.58.11.3751-3758.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowter C, McGee ZA. Evaluation of a technique for the demonstration of gonococci and other microorganisms in host cells. J. Clin. Pathol. 1976;29:433–437. doi: 10.1136/jcp.29.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D. The bacteriology of Gardnerella vaginalis. Scand J. Urol Nephrol. Suppl. 1984;86:41–55. [PubMed] [Google Scholar]
- Taylor-Robinson D, Carney FE. Growth and effect of Neisseria gonorrhoeae in organ cultures. Br J. Vener. Dis. 1973;49:435–440. doi: 10.1136/sti.49.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D, Hay PE. The pathogenesis of the clinical signs of bacterial vaginosis and possible reasons for its occurrence. Int. J. STD AIDS. 1997;8(Suppl 1):13–16. [Google Scholar]
- Taylor-Robinson AW, Borriello SP, Taylor-Robinson D. Identification and preliminary characterization of a cytotoxin isolated from Mobiluncus spp. Int. J. Exp. Pathol. 1993;74:357–366. [PMC free article] [PubMed] [Google Scholar]
- Ward ME, Watt PJ, Robertson JN. The human Fallopian tube: a laboratory model for gonococcal infection. J. Infect. Dis. 1974;129:650–659. doi: 10.1093/infdis/129.6.650. [DOI] [PubMed] [Google Scholar]


