Table 3.
Reaction of various indole based aldehydes with Rhodanine derivatives
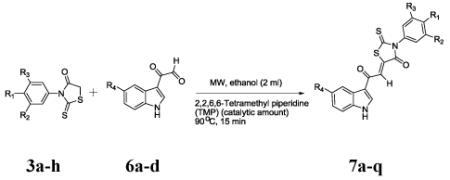
| Entry (%) |
R1 | R2 | R3 | R4 | product | Yielda,b |
|---|---|---|---|---|---|---|
| 1 | H | H | H | H | 7a | 95 |
| 2 | CH3 | H | H | H | 7b | 96 |
| 3 | CH3 | CH3 | H | H | 7c | 90 |
| 4 | CH3 | H | H | OCH3 | 7d | 93 |
| 5 | OCH3 | H | H | H | 7e | 95 |
| 6 | OCH3 | H | H | OCH3 | 7f | 92 |
| 7 | H | H | H | OCH3 | 7g | 94 |
| 8 | CN | H | H | OCH3 | 7h | 90 |
| 9 | CN | H | H | H | 7i | 91 |
| 10 | H | H | H | Cl | 7j | 89 |
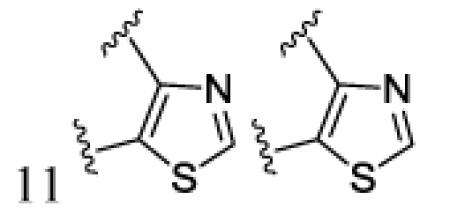
|
H | H | 7k | 95 | ||
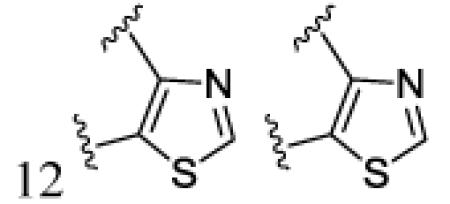
|
H | OCH3 | 7l | 93 | ||
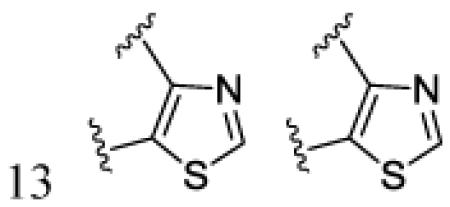
|
H | CN | 7m | 91 | ||
| 14 | OH | Br | Br | H | 7n | 90 |
| 15 | OH | Cl | Cl | H | 7o | 89 |
| 16 | OH | Br | Br | OCH3 | 7p | 96 |
| 17 | OH | Cl | Cl | OCH3 | 7q | 90 |
Isolated yield;
All the compounds were characterized by 1H NMR, 13C NMR, DEPT-135, IR and HRMS analysis.
