SUMMARY
In mammals, natriuretic peptides (NPs) lower blood pressure, reduce blood volume and broadly inhibit cardiovascular remodeling. NPs are often referred to as cardiac hormones, though they also have integral roles in regulating vascular tone, endothelial remodeling and inhibiting vascular smooth muscle cell hypertrophy. Two NPs [atrial (ANP) and C-type (CNP)] have been identified as endogenous constituents in the vasculature of mammals, though such a phenomenon has not previously been described in fishes. Here we describe the endogenous production of B-type NP (BNP) and CNP in multiple blood vessels of the rainbow trout. Western blot analysis showed pro-BNP and pro-CNP production in the efferent branchial artery, celiacomesenteric artery, ventral aorta and anterior cardinal vein. The detection of pro-BNP and pro-CNP was also supported by MALDI-TOF mass spectrometry analysis of NP-enriched tissue extracts. Although vascular pro-peptide levels of BNP and CNP were quantitatively quite comparable to those found in reference tissues (the atrium for BNP and brain for CNP), mRNA levels of these NPs in the vasculature were greatly reduced as determined by quantitative PCR. When the evolutionarily conserved vascular NP (CNP) was infused into un-anesthetized trout, it reduced central venous pressure and mean circulatory filling pressure. CNP also decreased cardiac output via a reduction in preload. The presence of endogenous NP production in the trout vasculature and potent in vivo hypotensive effects further support the numerous functional similarities between teleost and mammalian NP systems.
KEY WORDS: natriuretic peptide, vascular peptide, cardioprotection
INTRODUCTION
The natriuretic peptides (NPs) are a hormone family possessing integral cardiovascular and electrolyte homeostatic roles in vertebrates from cyclostomes to mammals (Donald and Trajanovska, 2006; Farrell and Olson, 2000; Johnson and Olson, 2008; Ramos and de Bold, 2006; Toop and Donald, 2004; Tsukada and Takei, 2006). The major members of this peptide family that are conserved between fishes and mammals are atrial NP (ANP), B-type NP (BNP) and C-type NP (CNP), whereas a fourth NP, ventricular NP (VNP), has been found only in a few teleosts (Inoue et al., 2005; Ventura et al., 2006). Among these NPs, to date only BNP has been found in cardiac tissue of species ranging from teleosts to mammals (Inoue et al., 2005; Johnson and Olson, 2008). These hormones are produced as pro-peptides and, following cleavage to their mature state, assume a disulfide-linked 17 amino acid ring structure with amino- and carboxy-terminal extensions of variable length. CNP, which lacks the carboxy-terminal extension, is widely considered the ancestral NP (Inoue et al., 2003a), and has been identified in multiple variant forms known as CNP1–4 in medaka, pufferfish and eel (Inoue et al., 2003a; Kawakoshi et al., 2004; Nobata et al., 2010). CNP is found in taxa from agnathans to humans, and appears to be ubiquitously expressed in the central nervous system, and to a lesser extent in the atrium (Arimura et al., 1991; Inoue et al., 2003b; Johnson and Olson, 2009b).
The functional significance of vascular NPs in mammals has been extensively reviewed (Ahluwalia et al., 2004; de Bold and de Bold, 2005; Woodard and Rosado, 2008). CNP and, to a lesser extent, ANP are produced in both endothelial and vascular smooth muscle cells (VSMCs), and inhibit VSMC proliferation, an effect mediated through natriuretic peptide receptor A (NPR-A; ANP-targeted receptor) and NPR-B (CNP-targeted receptor) (Furuya et al., 1993; Furuya et al., 1995; Itoh et al., 1990; Itoh et al., 1992; Yamahara et al., 2003). NPs also enhance endothelial cell regeneration in vitro and in vivo (Doi et al., 2001; Ohno et al., 2002). Delivery of CNP to vein grafts and vessels subjected to balloon injury (angioplasty) accelerates re-endothelialization but suppresses neointimal hyperplasia, both beneficial in maintaining the integrity of the manipulated vessel (Furuya et al., 1993; Ohno et al., 2002; Qian et al., 2002; Yamahara et al., 2003). Furthermore, ANP and CNP signaling via a paracrine–autocrine route likely contribute to local regulation of vascular tone (Ahluwalia et al., 2004; Chauhan et al., 2003). Indeed, in addition to anti-mitogenic actions, CNP also acts as a potent vasodilator in both mammals (Wei et al., 1994a; Wei et al., 1994b) and trout (Inoue et al., 2003b).
The teleost NP system bears many striking functional similarities to the cardiovascular anti-hypertrophic and volume-regulating NP system found in mammals (Farrell and Olson, 2000; Johnson and Olson, 2008; Johnson and Olson, 2009a). However, empirical information on extra-cardiac NP synthesis sites such as the vasculature in fish is limited. It is quite reasonable that the teleost NP system is the functional progenitor of the mammalian NP system. It has been argued that blood volume is the principal physiological stimulus of the NP system in both lineages (Farrell and Olson, 2000; Johnson and Olson, 2008), although some authors have suggested that fish NPs may primarily regulate salt excretion (Tsukada and Takei, 2006).
As the mammalian endothelium is a local source of CNP and CNP has been identified in trout brain and heart (Inoue et al., 2003b; Johnson and Olson, 2009b) and in fish plasma (Gary Anderson et al., 2005; Takei and Inoue, 2001), it is likely that fish possess a vascular NP component as well. Therefore, the principal aims of the present study were twofold: (1) to identify the endogenous production of NP(s) in the vasculature of rainbow trout, and (2) to assess the effects of CNP on the trout cardiovascular system in vivo.
MATERIALS AND METHODS
Animals
Adult rainbow trout (Oncorhynchus mykiss Walbaum 1792) of either sex were used for vascular protein, nucleotide applications, in vivo studies of cardiovascular function and vascular capacitance. All studies were conducted at the Indiana School of Medicine–South Bend. The trout were purchased from a local hatchery and maintained in circulating 2000 liter 12°C freshwater tanks under appropriate seasonal light:dark cycles. Fish were fed a maintenance diet of commercial trout pellets (Purina, St Louis, MO, USA) up to 48 h before experimentation. In all in vivo experiments, trout were anesthetized during surgery.
Antibody preparations and western blot analysis
Production of homologous trout BNP (tBNP) antibodies has been previously described (Johnson and Olson, 2009). Briefly, rainbow trout mature NPs (ANP, BNP, VNP and CNP) were aligned in order to determine regions of lowest similarity. A residue sequence in the carboxyl-terminus of BNP was identified as unique (VGKYNAKTR), and this sequence was commercially synthesized and used for tBNP polyclonal antibody production in New Zealand white rabbits by Genscript Corp. (Piscataway, NJ, USA). Antibodies used in detection of trout pro-CNP were generously donated by Dr Yoshio Takei (Ocean Research Institute, Tokyo, Japan) and prepared as previously described (Takei et al., 2001). These antibodies were raised against Japanese eel (Anguilla japonica) CNP, which bears a 95% homology to trout CNP (tCNP; 21 of 22 amino acids).
Homologous rainbow trout BNP antisera and eel CNP antisera were prepared for western blot analysis by affinity and negative affinity purification. Briefly, crude antisera were first direct-affinity purified using diaminodipropyl-amine (DADPA)-linked agarose beads coupled to the appropriate peptide using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC). Columns were washed with phosphate buffered saline (PBS) and an IgG elution buffer was applied to elute the bound antibodies. Antibodies were then negative-affinity purified using agarose beads coupled with NPs to which the antisera was not raised against. Collected antibody fractions were lyophilized and reconstituted at a stock concentration of 1 mg ml–1. The DADPA-linked agarose beads, EDC crosslinker and IgG elution buffer were purchased from Pierce (Rockford, IL, USA). Analysis of BNP and CNP antibody specificity and avidity was conducted using an antigen-capture, indirect ELISA method. Amine-binding, maleic-anhydride-coated 96 well plates (Pierce) were used in the adsorption of a range of purified NP concentrations (10–10–10–5). Following the addition of peptide, plates were incubated at room temperature for 2 h and then washed twice with PBS (pH 7.3). Blocking buffer composed of 2% (w/v) chicken ovalbumin and 0.05% Tween 20 was then added and the plates were incubated at 4°C overnight. The plates were then washed twice with PBS, and primary antibodies targeting tBNP or eel CNP (1:1000 dilutions in blocking buffer) were added to the wells and incubated for 2 h at room temperature. The plates were then washed four times with PBS, and secondary antibody was added. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (Sigma-Aldrich, St Louis, MO, USA) was diluted in blocking buffer (1:10,000) and added to each well. Plates were incubated with secondary antibody for 2 h at room temperature. A chromogenic substrate, 3,3′,5,5′-tetramethylbenzidine (TMB), was added to each well and incubated at room temperature for 30 min. The reaction was stopped by the addition of sulfuric acid, and the absorbance of each well was read at 450 nm using a colorimetric plate reader. These results are shown in supplementary material Fig. S1.
Atrial, brain and vascular protein extracts were prepared by homogenizing the atrium, brain, efferent branchial artery (EBA; third and fourth arch), celiacomesenteric artery (CMA), ventral aorta (VA) and anterior cardinal vein (ACV) in PBS (pH 7.5) with 5 mmol l–1 EDTA and 1 × HALT protease inhibitor cocktail (Pierce), 0.25% sodium deoxycholate and 1% Triton X-100. The crude lysates were centrifuged at 9850 g for 15 min at 4°C, protein concentration from the extract supernatants were determined using the Dc Protein Assay (BioRad Laboratories, Hercules, CA, USA). For SDS-PAGE separation, 50 μg of total protein were electrophoretically separated on a tris-tricine 12% polyacrylamide gel under denaturing conditions, then transferred to a nitrocellulose membrane (BioRad Laboratories) using a wet transfer unit. The membranes were blocked in 5% TBS-Tween-milk for 1 h at room temperature. Membranes were then probed with either trout BNP or eel CNP primary antibodies overnight at 4°C, whereas those for HRP-conjugated anti-rabbit IgG secondary antibody (Pierce) were incubated for 1 h at room temperature. Final primary antibody concentrations were 1 × 10–3 mg ml–1 (1:1000 dilution from stock 1 mg ml–1) for both BNP and CNP antibodies. Blots were incubated with enhanced chemiluminescence western blotting agent (Amersham Corp., Arlington Heights, IL, USA) for 1 min and exposed to X-ray film for 1–5 min. The calculated molecular weight of pro-CNP was approximately 14.6 kDa, while that of pro-BNP was 16.25 kDa. Predicted average molecular weights of pro-NPs were determined using the ExPASy Proteomics Server (http://ca.expasy.org/tools/pi_tool.html). To account for deviation in the amount of protein loaded, the same membranes were stripped with four washes of TBS-Tween and subsequently re-blotted for levels of β-tubulin to enable standardization [murine monoclonal anti-β tubulin antibody (Sigma-Aldrich)]. Western blots were quantitated by densitometry using ImageQuant software (Molecular Dynamics Inc., Sunnyvale, CA, USA).
Mass spectrometry
Atrial, brain, EBA and VA tissues were homogenized and protein extracts were quantitated as described above. Acetic acid was added to the protein samples to a final concentration of 1 mol l–1. Samples were then vortexed and centrifuged at 9850 g for 30 min at 4°C. The supernatant was then combined with two volumes of ice-cold acetone, vortexed and centrifuged at 9850 g for 30 min at 4°C; supernatants were then dried under an N2 stream. Protein pellets were then reconstituted with 1 mol l–1 acetic acid and applied to a C18 Sep-Pak (Waters Corporation, Milford, MA, USA). The C18 Sep-Paks were first activated with acetonitrile, washed with distilled water and then equilibrated with 1 mol l–1 acetic acid. Protein samples were applied to the Sep-Pak and washed with 1 mol l–1 acetic acid. Proteins were eluted with mixture of 60% acetonitrile:1 mol l–1 acetic acetic acid, then frozen and lyophilized. Protein pellets were reconstituted in a minimal volume of SDS-PAGE loading dye and then electrophoretically separated on a 12% Tris-Tricine polyacrylamide gel under denaturing conditions. Acrylamide gels were stained for 1 h in 0.03 g l–1 Coomassie Brilliant Blue R-250, 10% v/v acetic acid and 10% v/v isopropanol. Gels were then placed in a second staining solution containing 0.03 g l–1 Coomassie Brilliant Blue R-250, 10% v/v acetic acid for 1.5 h. Gels were then rinsed with water and used in MALDI mass spectrometry as described previously (Cohen and Chait, 1997). Briefly, protein bands migrating between 10 and 20 kDa were excised. The samples were extracted from the gel bands in a supersaturated solution of 3,5-dimethoxy-4-hydroxycinnamic acid matrix in 1:3:2 formic acid:water:2-propanol. The MALDI experiments were performed on a PerSeptive Biosystems (Framingham, MA, USA) Voyager-DE MALDI-TOF instrument in the positive-ion mode and using the linear delayed extraction method. The instrument is equipped with a nitrogen laser at 337 nm. The accelerating voltage was 25 kV and the delay was 100 ns. The grid voltage, guide wire voltage and laser power were optimized for each sample.
PCR amplification and DNA sequencing
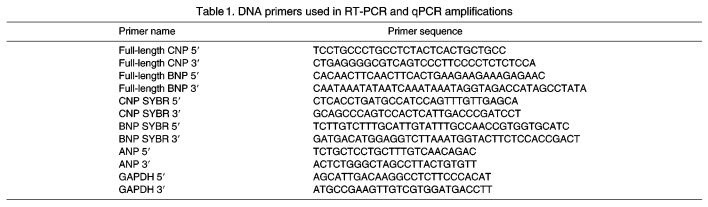
Total RNA was extracted from various tissues using Trizol reagent and, subsequently, poly-A mRNA was isolated using oligo-dT cellulose columns (Molecular Research Center, Cincinnati, OH, USA). Poly-A mRNA was then treated with amplification grade DNase I (Invitrogen, Carlsbad, CA, USA). Oligo-dT-primed, reverse-transcribed cDNA synthesis reactions were performed using the Promega RT System (Madison, WI, USA), according to the manufacturer's instructions. PCR amplification was used on the poly-A enriched cDNA using primers targeting the 5′ and 3′ untranslated regions (UTRs) of tBNP and tCNP (Table 1). PCR products were separated on a 1% agarose gel, excised and purified using QIAquick spin columns (Qiagen, Valencia, CA, USA). PCR products were sequenced using either the 5′ UTR or 3′ UTR primer on an ABI 3730XL capillary sequencer (Applied Biosystems, Foster City, CA, USA). Accession numbers are as follows: trout BNP, BAE19672; trout CNP-1, BAC44842; trout BNP intron 1, AB204713; trout BNP intron 2, AB204714.
Table 1.
DNA primers used in RT-PCR and qPCR amplifications
Quantitative PCR analysis
Forward and reverse primers for tBNP, tANP, tCNP and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were generated using MacVector software (Table 1) and were subsequently validated for use with real-time PCR by determining the optimal amplification efficiency and primer concentrations as described by the system manufacturer (Applied Biosystems). For real-time PCR, primers were added to 25 μl total reaction volume using reagents provided in the ABgene Absolute QPCR SYBR Green Mix (ABgene, Rochester, NY, USA). Final concentrations of the sense and antisense primers were determined for each primer pair based on optimal amplification efficiency. Reactions were carried out on an ABI 7700 Thermocycler (Applied Biosystems). Conditions were set to the following parameters: 2 min at 94°C followed by 40 cycles each for 15 s at 95°C, 1 min at 60°C, 1 min at 72°C. The cycle threshold (Ct; defined as the cycle number at which the fluorescence exceeds a threshold level) was determined for each reaction, and quantification was accomplished using the ΔΔCt method (Livak and Schmittgen, 2001). The target Ct was determined for each sample and then normalized to the GAPDH mRNA Ct from the same sample (GAPDH mRNA Ct subtracted from the target Ct yields the ΔCt). These values were then compared with control levels using the 2–ΔΔCt method and expressed as fold difference compared with an appropriate control sample.
Cardiovascular function
Methods for cannulation of the dorsal aorta, bulbus arteriosis and ductus Cuvier have been previously described in detail (Olson et al., 1997). Briefly, trout were anesthetized in benzocaine (ethyl-p-aminobenzoate; 1:12,000 w/v) before surgery. The dorsal aorta was cannulated percutaneously through the roof of the buccal cavity with heat-tapered polyethylene tubing (PE-60); the gills were not irrigated during this brief (∼1 min) procedure. Thereafter, gills were continuously irrigated with 4°C aerated water containing 1:24,000 w/v benzocaine. The pericardial cavity was exposed with a ventral incision and the ductus Cuvier was nonocclusively cannulated as previously described (Olson et al., 1997). The bulbus arteriosus was temporarily clamped at the ventriculobulbar junction and a short piece of silicone tubing was inserted through a small puncture in the central wall. The tubing was glued to the bulbus wall with cyanoacrylate glue and the distal end was attached to PE-90 tubing connected to a pressure transducer. After the cannula was inserted, the clamp was removed and a 2S Transonic flow probe (Transonic, Ithaca, NY, USA) was placed around the ventral aorta distal to the site of the cannula insertion and connected to a Transonic T101 or T206 flowmeter. The wound was closed with interrupted silk sutures and sealed with cyanoacrylate gel. Silk anchoring sutures were placed in the ventral skin, the inner margin of the pelvic fin, and the anal fin to secure venous and ventral aortic cannulas and the flow probe lead. All cannulas were filled with heparinized saline (100 USP units ml–1 heparin in 9.0 g l–1 NaCl) and connected to Gould P23 pressure transducers (Gould Instruments, Valley View, OH, USA). The fish were revived and placed in black plastic tubes suspended in the holding tanks. Experiments were conduced 24 h after surgery. An in-line four-way stopcock in the dorsal aortic cannula served as the site for infusion.
Analog pressure signals were recorded with Hewlett-Packard 7853A patient monitors (Palo Alto, CA, USA). Digitized signals of pressure and flow were collected at 0.1 s intervals, and 1 s averages were stored on a computer. The pressure transducers were calibrated with a water manometer, and the flowmeter was calibrated in situ at the end of the experiment by pump perfusion of the ventricle with 12°C saline at known flow rates. Heart rate (fH) was derived from the ventral aortic systolic pressure pulse or flow interval. Systemic vascular resistance (RS) was calculated by dividing the systemic pressure gradient (dorsal aortic pressure minus central venous pressure; PDA–PVEN) by cardiac output (OC), i.e. RS=(PDA–PVEN)/OC. Stroke volume (VS) was calculated from OC and fH, i.e. VS=OC h–1. Gill resistance (RG) was calculated in a similar manner, substituting the gill pressure gradient: RG=(PVA–PDA)/OC, where PVA is the ventral aortic pressure. Resting pressures and OC were monitored for 1–2 h before experimentation to ensure stability; control parameters were recorded for a minimum of 5 min before blood volume manipulation. Homologous tCNP was infused at 100 pmol kg–1 h–1, 1 nmol kg–1 h–1 and 10 nmol kg–1 h–1.
Vascular capacitance
Vascular capacitance curves were constructed from measurements on conscious trout in vivo using the ventral aorta occlusion method to establish zero-flow cardiac output as previously described (Olson and Hoagland, 2008; Zhang et al., 1998). Briefly, PDA and PVEN were measured in unanesthetized fish before and during occlusion of the ventral aorta. Transient inflation of the occluder (∼5 s) compressed the VA and produced a rapid decrease in PDA and increase in PVEN. PVEN was assumed to be equivalent to the mean circulatory filling pressure (MCFP) within 5–7 s. Vascular capacitance curves were obtained by measuring MCFP at 80, 90, 100, 110 and 120% of resting blood volume. Heparinized whole blood from a donor fish was used for volume expansion. All volume adjustments were made via the dorsal aorta cannula within 30 s prior to occlusion, and blood volume was restored to 100% within 30 s after occlusion. Only one capacitance curve was obtained from each fish. Vascular compliance was determined over the range of three blood volume intervals – 80–100, 90–110 and 100–120% – by regression analysis of the three consecutive pressure–volume data points within each interval. Because the actual blood volume was not measured when these experiments were conducted, we assumed a blood volume of 35 ml kg–1 body mass (Duff et al., 1987) and added or withdrew 3.5 and 7 ml kg–1 body mass. In the capacitance curve (Fig. 7C), MCFP by convention was treated as the independent variable, and the slope of the resultant volume–pressure line (Δvolume/Δpressure) was, therefore, equal to vascular compliance. The intercept of this line with the blood volume axis at MCFP=0 was assumed to be the percentage of the total blood volume in the unstressed compartment (Zhang et al., 1998).
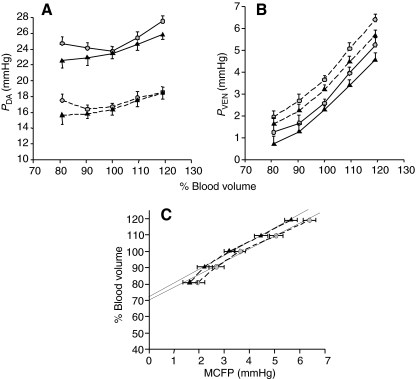
Fig. 7.
The effects of saline (circles) and CNP (triangles) infusions on (A) dorsal aortic pressure (PDA), (B) central venous pressure (PVEN) and (C) vascular capacitance. Blood volume was manipulated by infusion or withdrawal of blood in increments of 3.5 or 7 ml kg–1 body weight (estimated 10 and 20% of resting volume, respectively) and expressed as a percentage of total initial blood volume. Symbols connected by solid lines represent values obtained during uninterrupted cardiac output; symbols connected by dashed lines represent values obtained during zero-flow cardiac output. CNP infusion at the 80% blood volume resulted in a significant reduction in PDA (compared with saline infusion, *P<0.05) during zero-flow cardiac output (A). CNP infusion at the 120% blood volume resulted in a significant reduction in PVEN (compared with saline infusion, *P<0.05) during zero-flow cardiac output (B). Solid lines in C are regression lines for mean circulatory filling pressure (MCFP) between 90 and 100% blood volume. Their slope is equivalent to vascular compliance (2.9±0.3 ml mmHg–1 kg–1 for saline infusion and 3.1±0.3 ml mmHg–1 kg–1 for CNP infusion), and blood volume intercept at zero MCFP is the unstressed volume (70.0±2.7% for saline infusion and 72.1±2.3% for CNP infusion). Values are means ± s.e.m.
Data analysis
NP characterization studies were repeated a minimum of three times, unless otherwise stated. Summarized levels of NP mRNA and pro-NPs were expressed as fold-difference (mean ± s.e.m.) versus a designated reference sample such as atrium or brain (the value for the reference treatment was arbitrarily set at 1). Data were analyzed by t-test or by one-way ANOVA followed by the Fisher protected least significant difference multiple range test. Cardiovascular data was examined with Student's t-test, repeated-measures ANOVA and linear regression. Significance was assumed at P<0.05. Values are expressed as means ± s.e.m., or means + s.e.m. in many figures.
RESULTS
Vascular production of pro-CNP and pro-BNP in trout
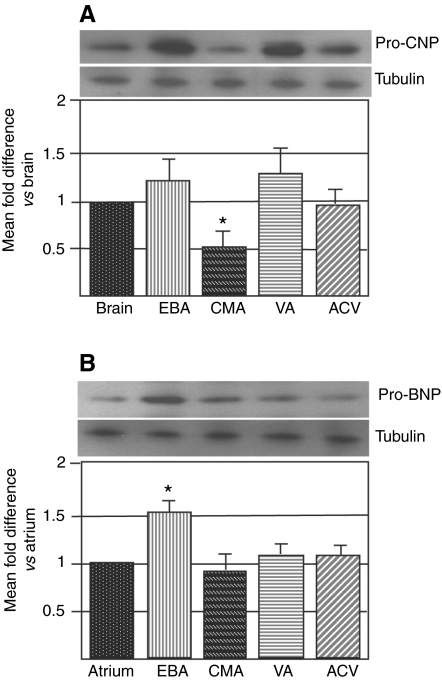
Using western analysis, pro-CNP peptide production was identified in the brain, EBA, CMA, VA and ACV (Fig. 1A). Pro-CNP peptide levels were significantly lower in the CMA than in the brain and other vessels. Pro-BNP peptide was found in the atrium, EBA, CMA, VA and ACV (Fig. 1B). Pro-BNP peptide levels were significantly higher in the EBA than the atrium.
Fig. 1.
Distribution of pro-NPs in trout tissues. (A) Pro-CNP is found in the brain, efferent branchial artery (EBA), celiacomesenteric artery (CMA), ventral aorta (VA) and anterior cardinal vein (ACV). Pro-CNP peptide levels were significantly lower in the CMA than the brain (N=5, *P<0.05). (B) Pro-BNP is found in the atrium, EBA, CMA, VA and ACV of trout. Pro-BNP peptide levels were significantly higher in the EBA than the atrium (N=5, *P<0.05). No other differences were found.
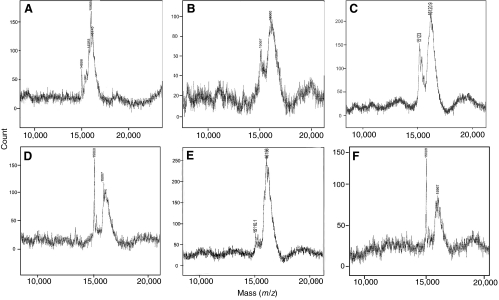
Proteomic evidence supportive of vascular pro-NP production (i.e. pro-BNP and pro-CNP) was generated by MALDI-TOF mass spectrometry (Fig. 2). Atrial, brain, EBA, CMA, VA and ACV protein extracts were enriched for pro-NPs by acetone extraction followed by C18 Sep-Pak purification. Subsequently, protein bands corresponding to immunoreactive pro-NPs (from western analysis) migrating at approximately 15 kDa were excised from polyacrylamide gels and purified for mass spectrometry. In the atrium, four protein mass peaks were identified from the trout atrium (Fig. 2A). These protein peaks correspond closely with trout pro-CNP (14,988 m/z), pro-ANP (15,763 m/z), pro-BNP (16,000 m/z) and pro-VNP (16,145 m/z). In the brain (Fig. 2B), two protein peaks were identified that are consistent with pro-CNP (15,057 m/z) and pro-BNP (16,000 m/z). Protein peaks consistent with pro-CNP and pro-BNP were also found in the EBA (Fig. 2C), CMA (Fig. 2D), VA (Fig. 2E) and ACV (Fig. 2F). The two m/z peaks between 15 and 16 kDa found in the vasculature are consistent with pro-CNP and pro-BNP, which were also seen in atrial and brain extracts.
Fig. 2.
Mass spectrometry (MALDI-TOF) proteomic analysis of NP-enriched protein extracts from atrial, brain, EBA and VA tissues. Following C18 Sep-Pak extraction and SDS-PAGE separation, proteins smaller than 20 kDa (corresponding to immunoreactive pro-NPs) were purified from acrylamide gels and analyzed by MALDI-TOF mass spectrometry. In the atrium (A), four peaks consistent with pro-CNP (molecular weight 14.471 kDa), pro-ANP (16.043 kDa), pro-BNP (16.124 kDa) and pro-VNP (16.663 kDa) were identified. In the brain (B), EBA (C), CMA (D), VA (E) and ACV (F), two peaks consistent with pro-CNP and pro-BNP were identified.
Tissue-dependent splice state of tBNP and tCNP
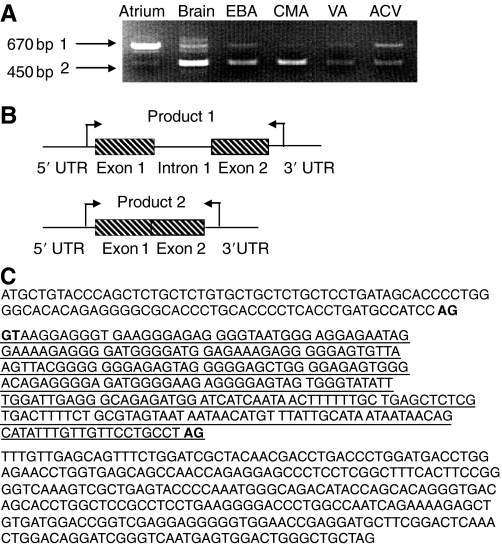
PCR amplification encompassing the 5′ and 3′ UTRs identified different splice states for both CNP and BNP mRNA. In the atrium, CNP was found predominantly in an unprocessed, pre-mRNA (intron-containing) state (Fig. 3A, product 1), whereas in the brain, EBA, CMA, VA and ACV, CNP was found in the fully processed state (Fig. 3A, product 2). Mature CNP mRNA is also seen in the atrium, though at considerably lower levels (Fig. 3A, product 2). A model was constructed showing the relative positions of the CNP exons and intron, and the tissues associated with each form (Fig. 3B). The CNP intronic sequence is underlined in Fig. 3C. The 5′ splice site boundary contains the consensus sequence AG (exon) GT (intron) and the 3′ splice site of the intron contained the consensus AG; both exon–intron boundaries are in bold (Fig. 3C).
Fig. 3.
RT-PCR amplification of CNP mRNA in trout tissues. (A) CNP mRNA in blood vessels (EBA, CMA, VA and ACV) is found in the mature, fully processed form and is indicated as the smaller PCR product (product 2). Atrial CNP mRNA is also seen in the mature form (product 2), though predominantly found in an unprocessed, pre-mRNA form containing CNP intron-1 and is indicated as the larger PCR product (product 1). (B) A model was constructed to show the relative positions of CNP exon 1, intron 1 and exon 2, and the tissues where the unspliced and spliced versions are found. Arrows indicate the relative docking positions of primers used in these amplifications. (C) The sequence of the CNP intron is underlined, flanked by CNP exons 1 and 2. Consensus 5′ and 3′ splice sites are in bold.
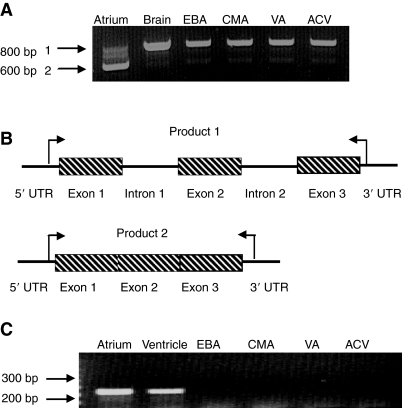
Atrial BNP mRNA was found predominantly in the mature (spliced) state, whereas in the brain, EBA, CMA, VA and ACV, BNP pre-mRNA was the predominate form, the latter containing BNP introns 1 and 2 (Fig. 4A). Mature BNP mRNA (spliced) is seen at considerably lower levels in the brain and vasculature (Fig. 4A,B, product 2). A model was constructed to show the relative positions of the BNP exons, introns and tissue(s) where each form was found (Fig. 4B). The trout vascular expression of a known mammalian vascular NP, ANP, was also examined by RT-PCR and found to be restricted to the heart (atrium and ventricle), with no detectable vascular expression (Fig. 4C).
Fig. 4.
RT-PCR amplification of BNP mRNA in trout tissues. (A) In the atrium, BNP mRNA is found predominantly in the mature, spliced form (PCR product 2) whereas in blood vessels (EBA, CMA, VA and ACV) the major form of BNP mRNA includes introns 1 and 2 and is indicated as the larger PCR product (product 1). Mature BNP mRNA is also seen in the brain and vasculature, though at much lower levels (product 2). (B) A model was constructed showing the relative positions of BNP exon 1, intron 1, exon 2, intron 2 and exon 3, and the tissues where the unspliced and spliced versions are found. Arrows indicate the relative docking positions of the primers used in these amplifications. (C) PCR amplification of trout ANP mRNA shows ANP expression is restricted to the heart (atrium and ventricle), with no detectable expression found in the vasculature.
Vascular expression of tCNP and tBNP mRNA
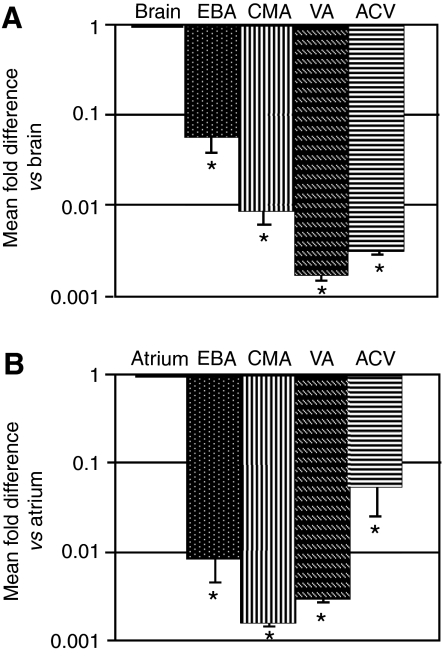
mRNA levels of both CNP and BNP (mature mRNA forms) were significantly reduced in the EBA, CMA, VA and ACV compared with the brain and atrium, respectively (Fig. 5).
Fig. 5.
Quantitative PCR analysis of CNP and BNP mRNA in various tissues of trout. (A) Relative abundance of CNP mRNA in the brain, EBA, CMA, VA and ACV. Levels of CNP mRNA were significantly lower in all vessels examined relative to the brain (N=4, *P<0.05). (B) Relative abundance of BNP mRNA levels in the atrium, EBA, CMA, VA and ACV. Levels of BNP mRNA were significantly lower in all vessels examined relative to the atrium (N=4, *P<0.05). No other differences were found.
Cardiovascular effects of homologous trout CNP
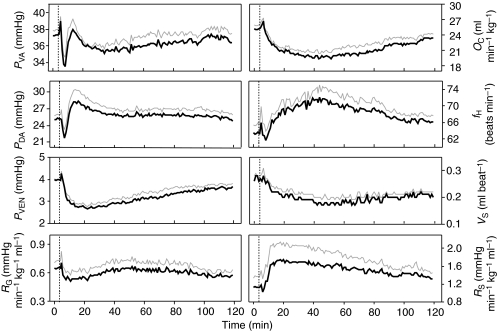
The in vivo effects of homologous trout CNP infusion at 10 nmol kg–1 h–1 are shown in Figs 6 and 7. CNP infusion at 100 pmol kg–1 h–1 and 1 nmol kg–1 h–1 produced similar but quantitatively lower responses (not shown). CNP had a striking and prolonged hypotensive effect on PVEN that developed within 10 min of infusion (Fig. 6). Upon CNP infusion, arterial pressure (PVA, PDA) initially dropped, then stabilized to near pre-infusion levels. CNP also reduced OC via a reduction in VS, whereas fH increased. RG fell following CNP infusion whereas RS increased (Fig. 6).
Fig. 6.
Cardiovascular effects of trout CNP (tCNP) infusion (10 nmol kg–1 h–1, administered after dotted line) on cardiovascular parameters in unanesthetized trout. The gray line is +1 s.e.m. (N=8). fH, heart rate (beats min–1); OC, cardiac output (ml min–1 kg–1); PVA, PDA and PVEN, ventral aortic pressure, dorsal aortic pressure and central venous pressure, respectively (mmHg); RG and RS, gill resistance and systemic resistance, respectively (mmHg min–1 kg–1 body weight ml–1); VS, stroke volume (ml beat–1).
The effects of CNP infusion on PDA, PVEN and vascular capacitance are shown in Fig. 7A–C, respectively. PDA, PVEN and MCFP were consistently lower in trout following CNP infusion compared with saline infusion (Fig. 7A–C). Although this trend was uniform throughout the blood volume status (80–120% blood volume), the efficacy of CNP in reducing PVEN and MCFP was significantly different in the volume-expanded states (110–120% blood volume; Fig. 7B). As shown in Fig. 7C, CNP infusion did not significantly affect vascular compliance or MCFP, though a trend in reduced MCFP at elevated blood volumes was apparent at 120% blood volume.
DISCUSSION
The present study shows that BNP and CNP are endogenously produced in the trout vasculature and the evolutionarily conserved vascular CNP has significant effects on PVEN and, ultimately, OC. Although endogenous synthesis of NPs has been reported in the mammalian vasculature (Hartmann et al., 2008; Potter et al., 2009) and a ubiquitous vascular expression of the guanylate cyclase-linked NP receptors A and B has been previously identified in trout (Johnson and Olson, 2009a), to our knowledge this is the first description of NP synthesis in the teleost vasculature.
Here we show mRNA and pro-peptide production of BNP and CNP in both pre-branchial (VA) and post-branchial (EBA, CMA and ACV) blood vessels. Pro-peptide levels of vascular BNP and CNP were quite comparable to levels found in reference tissues (brain for CNP, atrium for BNP), and only differed in specific instances (i.e. reduced CNP in the CMA and elevated BNP in the EBA). These peptides are likely stored as pro-hormones in the vasculature (as they are in the heart), because the processed (mature) mRNA levels of BNP and CNP in all blood vessels examined were significantly reduced compared with atrial BNP and brain CNP, respectively. In the atrium, which is a non-traditional production site for CNP (Inoue et al., 2003b; Johnson and Olson, 2009b), CNP mRNA was found in an un-spliced pre-mRNA form (intron-containing) where a previously unreported intronic sequence was identified (and submitted to GenBank as CNP intron-1). Alternatively, in both the brain and vasculature, CNP mRNA was found in the fully processed (intron-removed) form. Intron-containing (both intron-1 and intron-2) mRNA for BNP was found in both the brain and vasculature whereas BNP mRNA in the atrium was found in the completely processed (intron-removed) form. The identification of a CNP intronic sequence is consistent with other trout NP pre-mRNAs that also contain introns; trout BNP pre-mRNA contains two introns that were sequenced previously (accession numbers AB204713 and AB204714) and were also identified in this report (Fig. 4A,B). Trout ANP pre-mRNA contains one intronic sequence (accession number AB204710) and trout VNP pre-mRNA contains two intronic sequences (accession numbers AB204711 and AB204712).
NPs in mammals are well known for their vasorelaxant properties, as well as anti-mitogenic effects on endothelial and VSMCs both in vivo and in vitro (Itoh et al., 1990; Itoh et al., 1992; Pahm et al., 1997). It has been proposed that CNP in the mammalian vasculature maintains a substantial cytoprotective, anti-mitogenic, anti-atherogenic and vasorelaxant influence on the blood vessel wall, and that loss of endothelial-derived CNP may be an important contributor to the pathogenesis of inflammatory cardiovascular diseases such as atherosclerosis and restenosis (Ahluwalia et al., 2004). Other vascular stressors such as shear stress (blood flow) and stretch (blood pressure) have also been shown to augment vascular CNP production in mammals (Itoh and Nakao, 1999; Okahara et al., 1995; Zhang et al., 1999).
The aqueous, near-zero gravity and volume-expanding (freshwater) or volume-contracting (saltwater) piscine environments and the desiccating, orthostatic tetrapod environment present vastly different challenges to the vascular system. However, there are commonalities among vascular stressors between fish and mammals, including shear stress (Okahara et al., 1995; Zhang et al., 1999), atherosclerosis (Farrell, 2002; Farrell et al., 1986), as well as the necessity for active regulation of vascular tone and compliance with the means of affecting cardiac preload and afterload. It can be reasonably proposed that preload and cardiac filling pressure are likely even more important for determining VS in fish than in mammals, as the ejection fraction for fish hearts is quite high (80–100%) (Sandblom and Axelsson, 2007). Thus, it is likely that active regulation of venous tone is especially vital in euryhaline fishes that experience a wide range of osmotic stresses and subsequent perturbations in blood volume (Olson and Hoagland, 2008).
We did not examine the cytoprotective attributes of CNP but our observation of the prolonged hypotensive effect of CNP infusion on PVEN supports our hypothesis that one of the homeostatic functions of NPs in fish is to protect the heart from excessive preload and afterload (Farrell and Olson, 2000). The peripheral vasculature can adversely affect the heart through excessive preload, which promotes cardiac distension, or excessive afterload, which increases cardiac work. These excesses can be produced by venous and arterial vasoconstriction, respectively, and NP-mediated dilation can offset them (Farrell and Olson, 2000). It is evident from the present study that CNP can act similarly.
The main determinant of cardiac preload is PVEN (Olson and Farrell, 2006), which is reduced by CNP (Fig. 5) as well as ANP and VNP (Olson et al., 1997). Hence, endogenous vascular NP production is poised to provide rapid amelioration of elevated preload through reduction in PVEN. Because the fish gill is the primary site of NP clearance from the circulation in the trout (Duff and Olson, 1992), post-branchial release of NPs into the circulation would ensure venodilation. As we show, endogenous production of NPs, in post-branchial vessels (i.e. EBA, CMA and ACV), will effectively accomplish this. Pre-branchial NP synthesis in the vasculature, such as the VA and the heart (Johnson and Olson, 2009a; Johnson and Olson, 2009b), are likely modulators of RG (and to a lesser extent PVA), the primary determinants of afterload. Clearly, exogenous CNP can effectively reduce RG (Fig. 5) and thereby lower cardiac afterload. Thus, vascular NP production and release from both pre- and post-branchial vessels is ideally positioned to protect the pumping ability of the heart from excessive venous return (preload), as well as RS and RG (afterload).
We have previously shown that ANP and VNP infusion at 4.5 nmol kg–1 h–1 has qualitatively similar but quantitatively greater effects on cardiovascular parameters (pressure, VS and vascular resistance) than CNP infusion at 10 nmol kg–1 h–1 (Fig. 5) (Olson et al., 1997). A similar decrease in CNP efficacy compared with ANP, BNP and VNP has been observed in the eel cardiovascular system (Nobata et al., 2010), and this may be indicative of a generally lower CNP efficacy in fish. We also showed that rat ANP infusion into trout significantly increased unstressed blood volume and increased vascular compliance (Olson et al., 1997). Our inability to demonstrate a significant effect of CNP infusion on either of these parameters (Fig. 6) may also reflect this decreased efficacy.
As the evolutionary precursor to the mammalian cardiovascular system, teleosts provide basic yet very important empirical models to decipher the intricate characteristics and constraints that define the cardiovascular system throughout vertebrates. A strong argument has been made that the piscine NP system is principally responsive to volume, and hence the physiological progenitor to the mammalian NP system (Farrell and Olson, 2000; Johnson and Olson, 2008; Johnson and Olson, 2009a; Johnson and Olson, 2009b). The results from the present study further extend the multiple functional similarities between the piscine and mammalian NP systems and indicate that the production of NPs in the vasculature is a fundamental property of the NP system that is found throughout vertebrates.
Supplementary Material
ACKNOWLEDGEMENTS
The authors express their gratitude to Drs A. Johnson and D. Woods for technical assistance with many of the methods and use of equipment, and to Dr Y. Takei for generous donation the of eel CNP antibody used in this study. Thanks also to C. Gordon for invaluable secretarial assistance.
- ACV
- anterior cardinal vein
- ANP
- atrial natriuretic peptide
- BNP
- B-type natriuretic peptide
- CMA
- celiacomesenteric artery
- CNP
- C-type natriuretic peptide
- Ct
- cycle threshold
- DADPA
- diaminodipropyl-amine
- EBA
- efferent branchial artery
- EDC
- 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide
- fH
- heart rate
- GADPH
- glyceraldehyde 3-phosphate dehydrogenase
- HRP
- horseradish peroxidase
- MCFP
- mean circulatory filling pressure
- NP
- natriuretic peptide
- NPR-A
- natriuretic peptide receptor A
- NPR-B
- natriuretic peptide receptor B
- OC
- cardiac output
- PBS
- phosphate buffered saline
- PDA
- dorsal aortic pressure
- PVA
- ventral aortic pressure
- PVEN
- central venous pressure
- RG
- gill resistance
- RS
- systemic vascular resistance
- TBNP
- trout B-type natriuretic peptide
- TCNP
- trout C-type natriuretic peptide
- TMB
- 3,3′,5,5′-tetramethylbenzidine
- UTR
- untranslated region
- VA
- ventral aorta
- VNP
- ventricular natriuretic peptide
- VS
- stroke volume
- VSMC
- vascular smooth muscle cell
FOOTNOTES
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/214/16/2709/DC1
Supported in part by National Science Foundation grant nos. IBN 0235223 (K.R.O.) and IOS 0641436 (K.R.O.), and National Institutes of Health NRSA training grant NIH T32 HL 07692 (K.R.J.). Deposited in PMC for release after 12 months.
REFERENCES
- Ahluwalia A., MacAllister R. J., Hobbs A. J. (2004). Vascular actions of natriuretic peptides. Cyclic GMP-dependent and -independent mechanisms. Basic Res. Cardiol. 99, 83-89 [DOI] [PubMed] [Google Scholar]
- Arimura J. J., Minamino N., Kangawa K., Matsuo H. (1991). Isolation and identification of C-type natriuretic peptide in chicken brain. Biochem. Biophys. Res. Commun. 174, 142-148 [DOI] [PubMed] [Google Scholar]
- Chauhan S. D., Nilsson H., Ahluwalia A., Hobbs A. J. (2003). Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc. Natl. Acad. Sci. USA 100, 1426-1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. L., Chait B. T. (1997). Mass spectrometry of whole proteins eluted from sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Anal. Biochem. 247, 257-267 [DOI] [PubMed] [Google Scholar]
- de Bold A. J., de Bold M. L. (2005). Determinants of natriuretic peptide production by the heart: basic and clinical implications. J. Investig. Med. 53, 371-377 [DOI] [PubMed] [Google Scholar]
- Doi K., Ikeda T., Itoh H., Ueyama K., Hosoda K., Ogawa Y., Yamashita J., Chun T. H., Inoue M., Masatsugu K., et al. (2001). C-type natriuretic peptide induces redifferentiation of vascular smooth muscle cells with accelerated reendothelialization. Arterioscler. Thromb. Vasc. Biol. 21, 930-936 [DOI] [PubMed] [Google Scholar]
- Donald J. A., Trajanovska S. (2006). A perspective on the role of natriuretic peptides in amphibian osmoregulation. Gen. Comp. Endocrinol. 147, 47-53 [DOI] [PubMed] [Google Scholar]
- Duff D. W., Olson K. R. (1992). Atrial natriuretic peptide clearance receptors in trout: effects of receptor inhibition in vivo. J. Exp. Zool. 262, 343-346 [DOI] [PubMed] [Google Scholar]
- Duff D. W., Fitzgerald D., Kullman D., Lipke D. W., Ward J., Olson K. R. (1987). Blood volume and red cell space in tissues of the rainbow trout, Salmo gairdneri. Comp. Biochem. Physiol. 87A, 393-398 [DOI] [PubMed] [Google Scholar]
- Farrell A. P. (2002). Coronary arteriosclerosis in salmon: growing old or growing fast? Comp. Biochem. Physiol. 132A, 723-735 [DOI] [PubMed] [Google Scholar]
- Farrell A. P., Olson K. R. (2000). Cardiac natriuretic peptides: a physiological lineage of cardioprotective hormones? Physiol. Biochem. Zool. 73, 1-11 [DOI] [PubMed] [Google Scholar]
- Farrell A. P., Saunders R. L., Freeman H. C., Mommsen T. P. (1986). Arteriosclerosis in Atlantic salmon. Effects of dietary cholesterol and maturation. Arteriosclerosis 6, 453-461 [DOI] [PubMed] [Google Scholar]
- Furuya M., Aisaka K., Miyazaki T., Honbou N., Kawashima K., Ohno T., Tanaka S., Minamino N., Kangawa K., Matsuo H. (1993). C-type natriuretic peptide inhibits intimal thickening after vascular injury. Biochem. Biophys. Res. Commun. 193, 248-253 [DOI] [PubMed] [Google Scholar]
- Furuya M., Miyazaki T., Honbou N., Kawashima K., Ohno T., Tanaka S., Kangawa K., Matsuo H. (1995). C-type natriuretic peptide inhibits intimal thickening after vascular injury. Ann. N. Y. Acad. Sci. 748, 517-523 [DOI] [PubMed] [Google Scholar]
- Gary Anderson W., Hyodo S., Tsukada T., Meischke L., Pillans R. D., Good J. P., Takei Y., Cramb G., Franklin C. E., Hazon N. (2005). Sequence, circulating levels, and expression of C-type natriuretic peptide in a euryhaline elasmobranch, Carcharhinus leucas. Gen. Comp. Endocrinol. 144, 90-98 [DOI] [PubMed] [Google Scholar]
- Hartmann M., Skrybain B. V., Muller T., Gazinski A., Schroter J., Gassner B., Nikolaev V. O., Bunemann M., Kuhn M. (2008). Alternative splicing of the guanylyl cyclase-A receptor modulates atrial natriuretic peptide signaling. J. Biol. Chem. 283, 28313-28320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Naruse K., Yamagami S., Mitani H., Suzuki N., Takei Y. (2003a). Four functionally distinct C-type natriuretic peptides found in fish reveal evolutionary history of the natriuretic peptide system. Proc. Natl. Acad. Sci. USA 100, 10079-10084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Russell M. J., Olson K. R., Takei Y. (2003b). C-type natriuretic peptide of rainbow trout (Oncorhynchus mykiss): primary structure and vasorelaxant activities. Gen. Comp. Endocrinol. 130, 185-192 [DOI] [PubMed] [Google Scholar]
- Inoue K., Sakamoto T., Yuge S., Iwatani H., Yamagami S., Tsutsumi M., Hori H., Cerra M. C., Tota B., Suzuki N., et al. (2005). Structural and functional evolution of three cardiac natriuretic peptides. Mol. Biol. Evol. 22, 2428-2434 [DOI] [PubMed] [Google Scholar]
- Itoh H., Nakao K. (1999). Vascular stress response and endothelial vasoactive factors for vascular remodelling. Diabetes Res. Clin. Pract. 45, 83-88 [DOI] [PubMed] [Google Scholar]
- Itoh H., Pratt R. E., Dzau V. J. (1990). Atrial natriuretic polypeptide inhibits hypertrophy of vascular smooth muscle cells. J. Clin. Invest. 86, 1690-1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H., Pratt R. E., Ohno M., Dzau V. J. (1992). Atrial natriuretic polypeptide as a novel antigrowth factor of endothelial cells. Hypertension 19, 758-761 [DOI] [PubMed] [Google Scholar]
- Johnson K. R., Olson K. R. (2008). Comparative physiology of the piscine natriuretic peptide system. Gen. Comp. Endocrinol. 157, 21-26 [DOI] [PubMed] [Google Scholar]
- Johnson K. R., Olson K. R. (2009a). Responses of the trout cardiac natriuretic peptide system to manipulation of salt and water balance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1170-R1190 [DOI] [PubMed] [Google Scholar]
- Johnson K. R., Olson K. R. (2009b). The response of non-traditional natriuretic peptide production sites to salt and water manipulations in the rainbow trout. J. Exp. Biol. 212, 2991-2997 [DOI] [PubMed] [Google Scholar]
- Kawakoshi A., Hyodo S., Inoue K., Kobayashi Y., Takei Y. (2004). Four natriuretic peptides (ANP, BNP, VNP and CNP) coexist in the sturgeon: identification of BNP in fish lineage. J. Mol. Endocrinol. 32, 547-555 [DOI] [PubMed] [Google Scholar]
- Livak K., Schmittgen T. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402-408 [DOI] [PubMed] [Google Scholar]
- Nobata S., Ventura A., Kaiya H., Takei Y. (2010). Diversified cardiovascular actions of six homologous natriuretic peptides (ANP, BNP, VNP, CNP1, CNP3, and CNP4) in conscious eels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1549-R1559 [DOI] [PubMed] [Google Scholar]
- Ohno N., Itoh H., Ikeda T., Ueyama K., Yamahara K., Doi K., Yamashita J., Inoue M., Masatsugu K., Sawada N., et al. (2002). Accelerated reendothelialization with suppressed thrombogenic property and neointimal hyperplasia of rabbit jugular vein grafts by adenovirus-mediated gene transfer of C-type natriuretic peptide. Circulation 105, 1623-1626 [DOI] [PubMed] [Google Scholar]
- Okahara K., Kambayashi J., Ohnishi T., Fujiwara Y., Kawasaki T., Monden M. (1995). Shear stress induces expression of CNP gene in human endothelial cells. FEBS Lett. 373, 108-110 [DOI] [PubMed] [Google Scholar]
- Olson K. R., Farrell A. P. (2006). The cardiovascular system, chapter 1. In The Physiology of Fishes (ed. Evans D. H., Claiborne J. B.), pp. 119-152 Boca Raton, FL: Taylor and Francis; [Google Scholar]
- Olson K. R., Hoagland T. M. (2008). Effects of freshwater and saltwater adaptation and dietary salt on fluid compartments, blood pressure, and venous capacitance in trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1061-R1067 [DOI] [PubMed] [Google Scholar]
- Olson K. R., Conklin D. J., Farrell A. P., Keen J. E., Takei Y., Weaver L., Jr, Smith M. P., Zhang Y. (1997). Effects of natriuretic peptides and nitroprusside on venous function in trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 273, R527-R539 [DOI] [PubMed] [Google Scholar]
- Pahm I., Sediame S., Maistre G., Roudot-Thoraval F., Chabrier P. E., Carayon A., Adnot S. (1997). Renal and vascular effects of C-type and atrial natriuretic peptides in humans. Am J. Physiol. 273, R1457-R1464 [DOI] [PubMed] [Google Scholar]
- Potter L. R., Yoder A. R., Flora D. R., Antos L. K., Dickey D. M. (2009). Natriuretic peptides: their structure, receptors, physiologic functions and therapeutic applications. Handb. Exp. Pharmacol. 191, 341-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J. Y., Haruno A., Asada Y., Nishida T., Saito Y., Matsuda T., Ueno H. (2002). Local expression of C-type natriuretic peptide suppresses inflammation, eliminates shear stress-induced thrombosis, and prevents neointima formation through enhanced nitric oxide production in rabbit injured carotid arteries. Circ. Res. 91, 1063-1069 [DOI] [PubMed] [Google Scholar]
- Ramos H., de Bold A. J. (2006). Gene expression, processing, and secretion of natriuretic peptides: physiologic and diagnostic implications. Heart Fail. Clin. 2, 255-268 [DOI] [PubMed] [Google Scholar]
- Sandblom E., Axelsson M. (2007). The venous circulation: a piscine perspective. Comp. Biochem. Physiol. 148A, 785-801 [DOI] [PubMed] [Google Scholar]
- Takei Y., Inoue K. (2001). Enhanced expression and release of C-type natriuretic peptide in freshwater eels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1727-R1735 [DOI] [PubMed] [Google Scholar]
- Takei Y., Inoue K., Ando K., Ihara T., Katafuchi T., Kashiwagi M., Hirose S. (2001). Enhanced expression and release of C-type natriuretic peptide in freshwater eels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1727-R1735 [DOI] [PubMed] [Google Scholar]
- Toop T., Donald J. A. (2004). Comparative aspects of natriuretic peptide physiology in non-mammalian vertebrates: a review. J. Comp. Physiol. B 174, 189-204 [DOI] [PubMed] [Google Scholar]
- Tsukada T., Takei Y. (2006). Integrative approach to osmoregulatory action of atrial natriuretic peptide in seawater eels. Gen. Comp. Endocrinol. 147, 31-38 [DOI] [PubMed] [Google Scholar]
- Ventura A., Kawakoshi A., Inoue K., Takei Y. (2006). Multiple natriuretic peptides coexist in the most primitive extant ray-finned fish, bichir Polypterus endlicheri. Gen. Comp. Endocrinol. 146, 251-256 [DOI] [PubMed] [Google Scholar]
- Wei C. M., Hu S., Miller V. M., Burnett J. C., Jr (1994a). Vascular actions of C-type natriuretic peptide in isolated porcine coronary arteries and coronary vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 205, 765-771 [DOI] [PubMed] [Google Scholar]
- Wei C. M., Kim C. H., Khraibi A. A., Miller V. M., Burnett J. C., Jr (1994b). Atrial natriuretic peptide and C-type natriuretic peptide in spontaneously hypertensive rats and their vasorelaxing actions in vitro. Hypertension 23, 903-907 [DOI] [PubMed] [Google Scholar]
- Woodard G. E., Rosado J. A. (2008). Natriuretic peptides in vascular physiology and pathology. Int. Rev. Cell Mol. Biol. 268, 59-93 [DOI] [PubMed] [Google Scholar]
- Yamahara K., Itoh H., Chun T. H., Ogawa Y., Yamashita J., Sawada N., Fukunaga Y., Sone M., Yurugi-Kobayashi T., Miyashita K., et al. (2003). Significance and therapeutic potential of the natriuretic peptides/cGMP/cGMP-dependent protein kinase pathway in vascular regeneration. Proc. Natl. Acad. Sci. USA 100, 3404-3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Weaver L., Jr, Ibeawuchi A., Olson K. R. (1998). Catecholaminergic regulation of venous function in the rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 274, R1195-R1202 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Xiao Z., Diamond S. L. (1999). Shear stress induction of C-type natriuretic peptide (CNP) in endothelial cells is independent of NO autocrine signaling. Ann. Biomed. Eng. 27, 419-426 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.