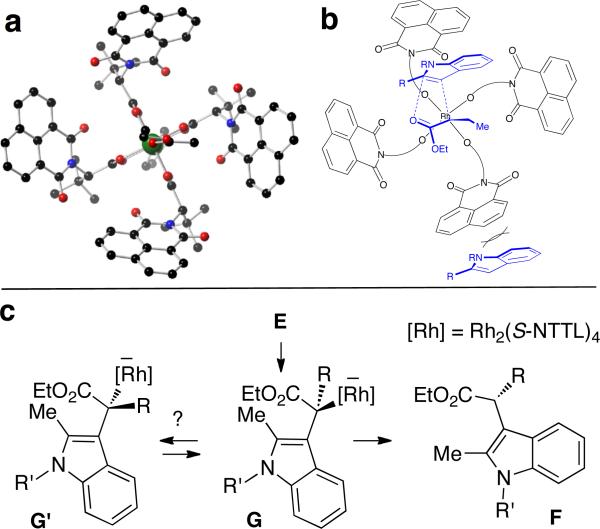
Figure 2.
(a) x-ray crystal structure of Rh2(S-NTTL)4. (b) Asymmetric induction may be explained by approach of the indole to the si-face of the Rh-carbene, with subsequent aromatization and stereoretentive protonation. (c) Alternatively, asymmetric induction may occur via dynamic kinetic resolution, provided that equilibrium between diastereomeric Rh-enolates G and G’ is fast relative to the rate of protonation.