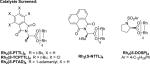
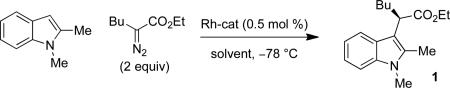
Table 1.
Selected Enantioselective Indole Functionalization Optimization Experimentsa
 | ||||
|---|---|---|---|---|
| entry | solvent | catalyst | yield(%)b | ee(%)c |
| 1 | PhMe | Rh2(S-PTTL)4 | 73 | 85 |
| 2 | PhMe | Rh2(S-TCPTTL)4 | 20 | 76 |
| 3 | PhMe | Rh2(S-NTTL)4 | 95 | 95 |
| 4 | PhMe | Rh2(R-PTAD)4 | 54 | –91 |
| 5 | PhMe | Rh2(S-DOSP)4 | 24 | 20 |
| 6 | CH2Cl2 | Rh2(S-NTTL)4 | 56 | 92 |
| 7d | PhMe | Rh2(S-NTTL)4 | 36 | 85 |
Conditions: indole (0.2 M), Rh-cat (0.5 mol %) at –78 °C, α-diazoester (0.67 M) added via syringe pump.
Isolated yield.
Determined by chiral HPLC analysis.
Reaction run at 0 °C. Optimal conditions in bold.