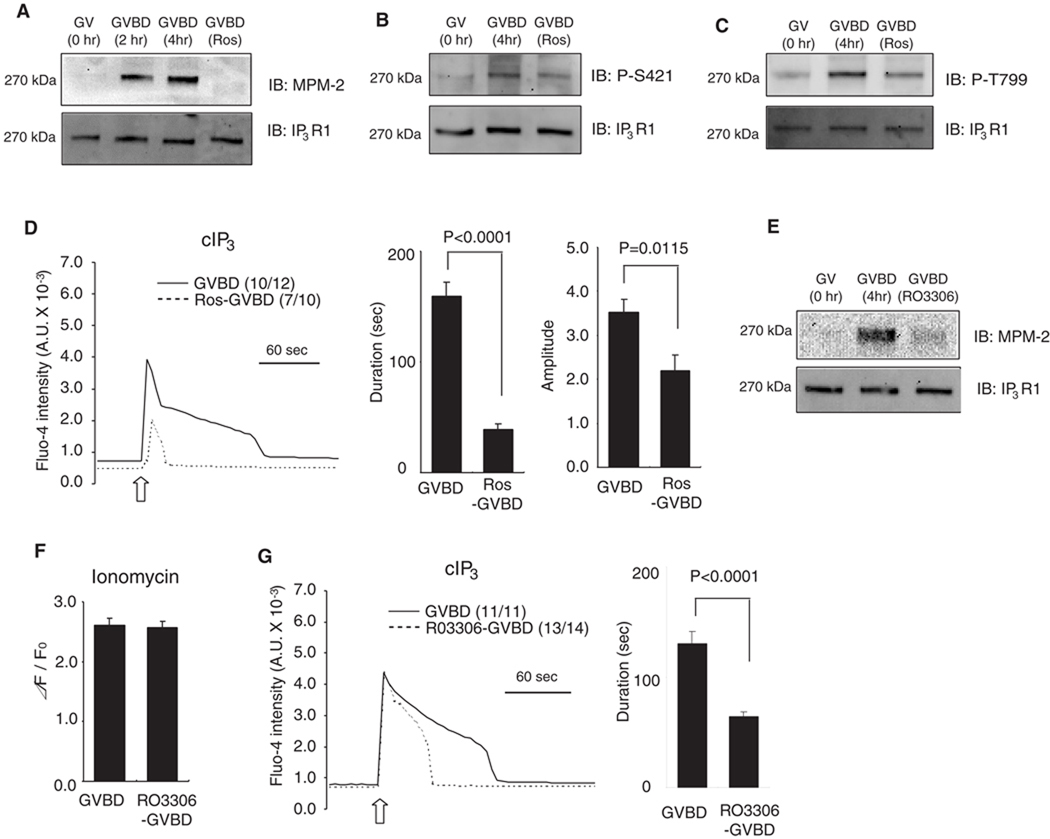
Fig. 6.
Phosphorylation-dependent sensitization of IP3R1 during oocyte maturation. (A–D) Inhibition of cdk1 activity was achieved by the addition of roscovitine (Ros). GV oocytes were allowed to undergo GVBD and 50 µM Ros was added 2 hr after the start of maturation. Lysates from GV, GVBD and Ros-treated GVBD oocytes were probed with MPM-2 (A), phospho-S421 (B) and phospho-T779 (C) antibodies (upper panel), and re-probed with IP3R1 antibody (lower panel), as described above. A representative result of 2–3 independent experiments with similar results is shown. (D) IP3-induced Ca2+ release using cIP3 was measured in control (solid line) and Ros-treaded (dashed line) oocytes. A 0.001 sec UV pulse was applied to oocytes (open arrow), number of oocytes responding to cIP3 is shown in parentheses. The bar graphs show comparison of the duration and amplitude of cIP3-caused Ca2+ release. (E–G) RO3306, another cdk1 inhibitor was used to block MPF activity. (E) IP3R1 MPM-2 (upper panel) and IP3R1 reactivity (lower panel) in GV, GVBD and RO3306-treated GVBD oocytes. (F) [Ca2+]ER was examined in control (n=9) and RO3306-treated GVBD oocytes (n=10) by the addition of 2 µM ionomycin in Ca2+ free medium. (G) IP3-induced Ca2+ release using cIP3 was measured in control (solid line) and Ros-treaded (dashed line) GVBD oocytes and induced by the application of a 0.001 sec UV pulse (open arrow). The number of oocytes responding to cIP3 is shown in parentheses. The bar graph shows the comparisons of the duration of the cIP3-induced Ca2+ release.