FIGURE 5. MtSmtB binds its own operator-promoter and responds to zinc in cells grown in Chelex-treated Sauton medium.
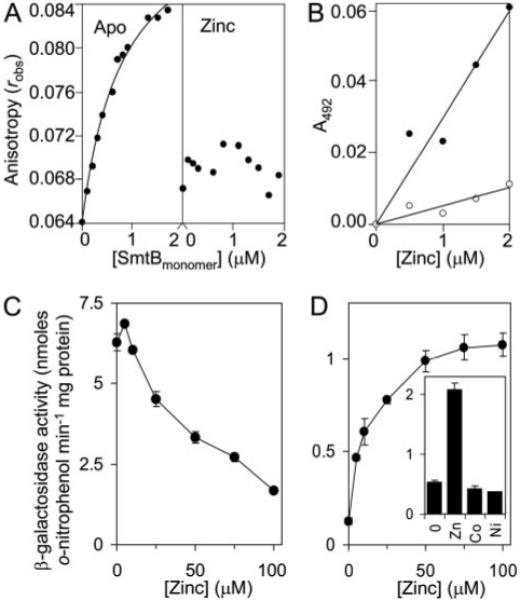
A, MtSmtB (50 μm) was incubated overnight with either 1 mm EDTA (left) or 20 μm Zn(II) (right) and increasing concentrations added to 6-hexachloroflourescein-labeled PMtSmtB 30-mer (5 nm) in the presence of 1 mm EDTA or 35 μm ZnCl2 (final [Zn(II)] = 35–35.84 μm), respectively, under anaerobic conditions and anisotropy, robs, measured. The curve represents a 1:1 binding model, KMtSmtB = 0.81 μm. B, microtiter plate assays of Zn(II)-PAR formation (400 μm PAR), measured at 492 nm and zeroed against apo-PAR, as a function of [Zn(II)] in the absence (closed symbols) or presence (open symbols) of MtSmtB (2 μm). C and D, β-galactosidase activity measured in M. smegmatis mc2155 containing MtsmtB-regulated lacZ grown up to inhibitory [Zn(II)] in Middlebrook 7H9 medium (C) or metal-depleted Sauton medium (D). Inset, β-galactosidase activity in cells grown with no metal supplement or with maximum permissive concentrations of Zn(II) (70 μm), Co(II) (15 μm), or Ni(II) (30 μm).
