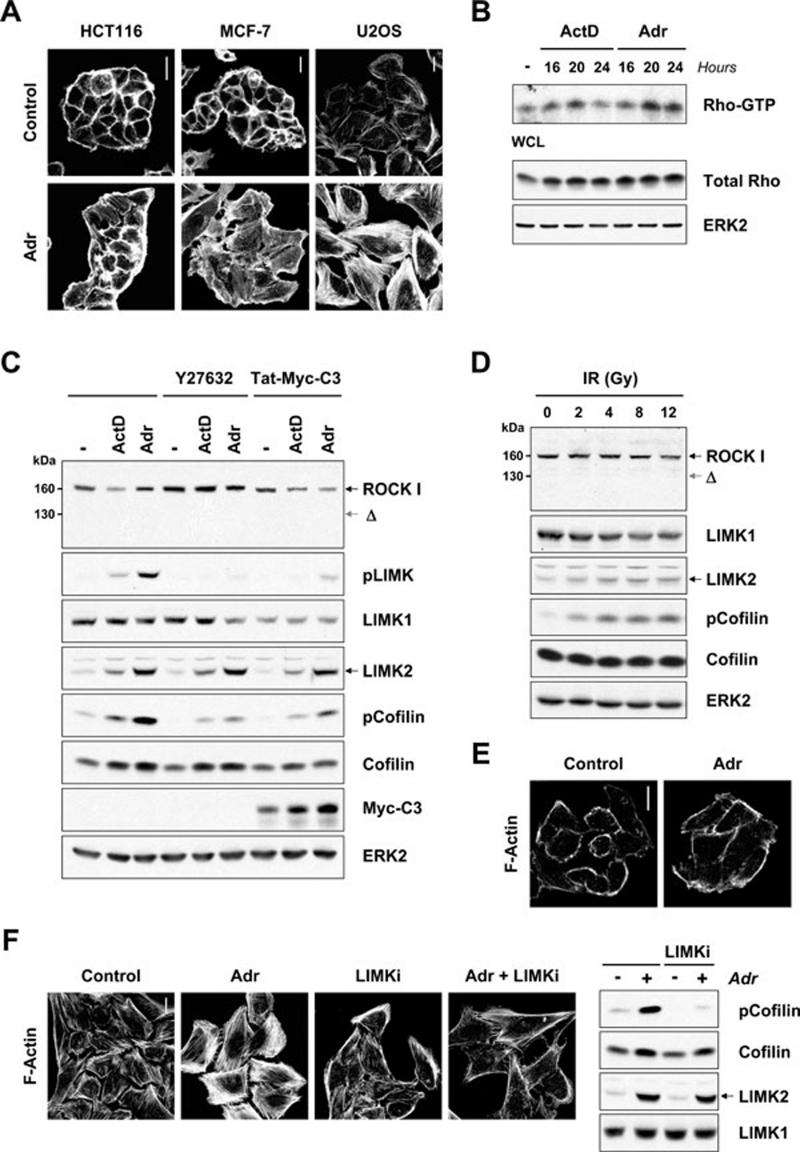
Figure 1.
Genotoxic stress activates the Rho-ROCK-LIMK pathway. (A) Genotoxic stress induces actin stress fibers. HCT116, MCF-7 or U2OS cells were treated with adriamycin (Adr) (0.2 μg/ml) for 24 h. Cells were then fixed and F-actin structures visualized with Texas Red-conjugated phalloidin. Scale bars = 20 μm. (B) Genotoxic stress activates Rho. Active Rho-GTP was affinity purified using recombinant Rhotekin Rho-binding domain from MCF-7 cells treated with actinomycin-D (ActD; 2 nM) or Adr (0.2 μg/ml) for 16, 20 or 24 h. ERK2 immunoblotting showed equivalent protein loading. (C) Genotoxic stress leads to LIMK and cofilin phosphorylation. MCF-7 cells were treated with ActD (2 nM) or Adr (0.2 μg/ml) in the presence or absence of Y-27632 (10 μM) or Tat-Myc-C3 (80 μg/ml) for 24 h. Whole cell lysates were immunoblotted with antibodies against ROCK1, phospho-LIMK1 (Thr508)/LIMK2 (Thr505), LIMK1, LIMK2, phosphocofilin (Ser3), cofilin or Myc-epitope. LIMK phosphorylation was blocked by ROCK inhibitor Y-27632 or Rho inhibitor Tat-Myc-C3. Equivalent protein loading was confirmed by ERK2 immunoblotting. (D) Ionizing radiation activates LIMK and leads to cofilin phosphorylation. MCF-7 cells were treated with or without IR (2, 4, 8 and 12 Gy) and whole cell lysates prepared after 24 h. Lysates were immunoblotted with antibodies against ROCK1, LIMK1, LIMK2, phosphocofilin (Ser3) and cofilin. ERK immunoblotting indicated equivalent protein loading. (E) Genotoxic stress does not promote stress fiber formation in cells with mutant p53. MDA-MB-231 cells were treated with Adr (0.2 μg/ml) for 24 h. Cells were then fixed and F-actin structures visualized with Texas Red-conjugated phalloidin. Scale bar = 20 μm. (F) LIMKi inhibits adriamycin-induced actin stress fiber formation and cofilin phosphorylation. U2OS cells were treated with Adr (0.2 μg/ml) in the presence or absence of LIMKi (10 μM) for 24 h. Cells were then fixed and F-actin structures visualized with Texas Red-conjugated phalloidin. Scale bar = 20 μm. MCF-7 cells were treated with Adr (0.2 μg/ml) in the presence or absence of LIMKi (3 μM) for 24 h. Whole cell lysates were immunoblotted with antibodies against phosphocofilin (Ser3), cofilin and LIMK2. Equivalent protein loading was confirmed by LIMK1 immunoblotting.