Table 2. Cumulative treatment failure (primary end-point) and accompanying reasons by study arm for 812 subjects randomised to doctor or nurse monitored antiretroviral therapy in the CIPRA-SA trial in South Africa†.
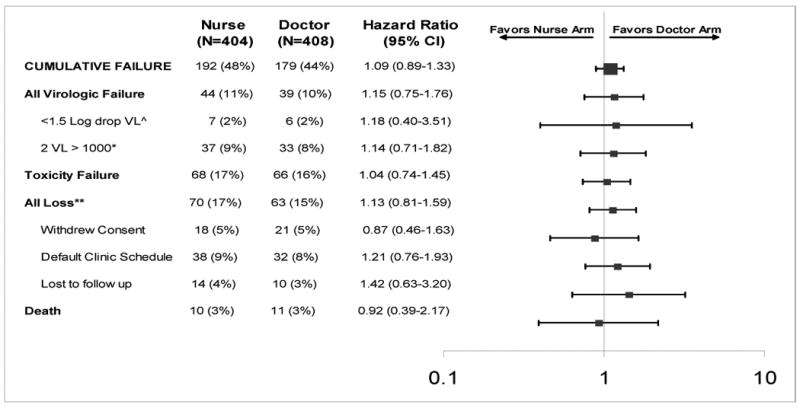
|
The two arms of the study were compared using a composite end-point or cumulative treatment failure. The composite consisted of each of the reasons listed below.
Early virologic failure was defined when a participant failed to demonstrate a serologic viral load decline of more than 1.5 logarithm within 12 weeks of initiating treatment.
Late virologic failure was defined as rebound in viral load from undetectable to more than 1000 copies/ml confirmed within one month.
Any loss was defined as: 1) withdrawn consent was if the patient withdrew from participating in the study for whatever reason and represents in most cases a transfer away from the site to another geographic location and clinic; 2) defaulting clinic schedule was a protocol defined measure of adherence to the clinic schedule, any participant who missed three consecutive visits was considered to be defaulting; (3) Loss to follow-up was consider if a participant did not return to the clinic for three consecutive clinic visits and could not be traced.
