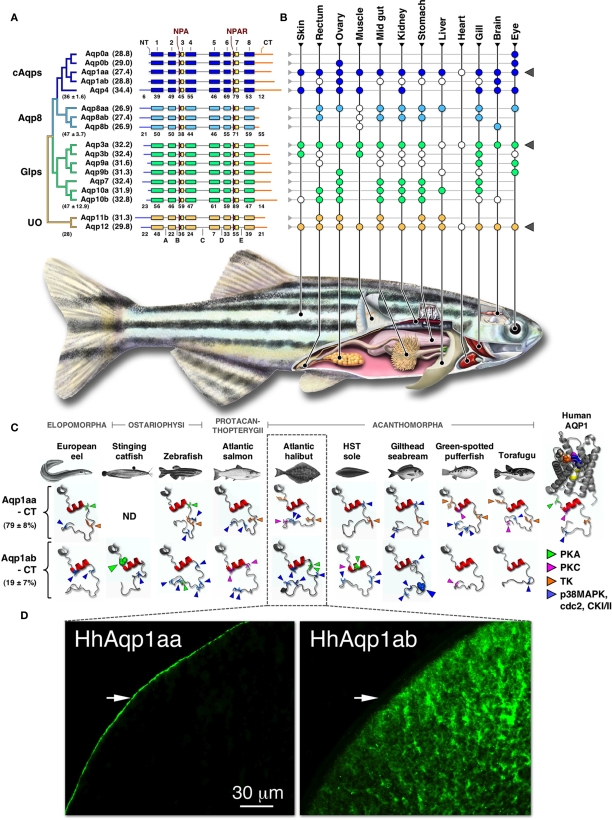
Figure 1.
Phylogeny, structure, and expression of teleost aquaporins. (A) Bayesian midpoint rooted tree of the zebrafish aquaporin superfamily with predicted molecular masses of the deduced proteins given in parentheses. Linear-scale alignment of the superfamily shows the secondary structural conservation of α-helices 1–8 and the diversity of the N- (NT) and C-termini (CT) within each subfamily. The canonical Asn-Pro-Ala (NPA) and Asn-Pro-Ala-Arg (NPAR) motifs that are present in most paralogs are shown upstream of hemi-helices 3 and 7, respectively. Loops A–E are indicted below the unorthodox (UO) aquaporins. Identity values of the full-length (in parentheses ± SD), the α-helical, and the terminal subdomains of the classical aquaporins (cAqps), aquaporin 8 (Aqp8), aquaglyceroporins (Glps), and the UO aquaporins are shown below each group. (B) Summarized view of the tissue expression pattern of zebrafish aquaporins. Colored dots refer to the distribution in adults, while white dots represent data reported for other species of teleost. In some species the anterior intestine is indicated by the stomach, while the brain includes chemosensory and mechanosensory organs. Gray triangles to the right highlight ubiquitous or semi-ubiquitous expression patterns. (C) Three-dimensional cartoon renders of the cytoplasmic C-terminal regions of teleost Aqp1aa and -1ab compared to Human AQP1 (1H6I). Models were generated using ModWeb and rendered with MacPyMOL. For the human AQP1 channel, the NPA motifs are highlighted in yellow and the ar/R constriction residues (Phe, magenta; His, wheat; Cys, Orange; Arg, blue) are shown as spacefill. All proteins retain a tertiary helix (red H9) but otherwise fold as disordered loops extending intracellularly from α-helix 8. Putative phosphorylation sites are indicated as protein kinase A (PKA), protein kinase C (PKC), tyrosine kinase (TK), p38 mitogen-activated protein kinase (p38MAPK), cell division cycle 2 (cdc2), or casein type kinase (CKI/II). Phosphorylation sites known to influence membrane trafficking are rendered as spacefill for the stinging catfish (green) and gilthead seabream (blue). Identity (±SD) of the aligned teleost C-termini are given to the left. ND: no data. (D). Localized cellular expression of Atlantic halibut (Hippoglossus hippoglossus) HhAqp1aa and -1ab (green) following ex vivo injection of transcripts in Xenopus laevis oocytes. HhAqp1aa is constitutively expressed in the plasma membrane (white arrows), while HhAqp1ab is retained in intracellular vesicles. Data for this figure are recompiled from Cerdà and Finn (2010), Sun et al. (2010), Tingaud-Sequeira et al. (2008, 2010), Tipsmark et al. (2010), Chaube et al. (2011), Zapater et al. (2011), and Zichichi et al. (2011).