Figure 3.
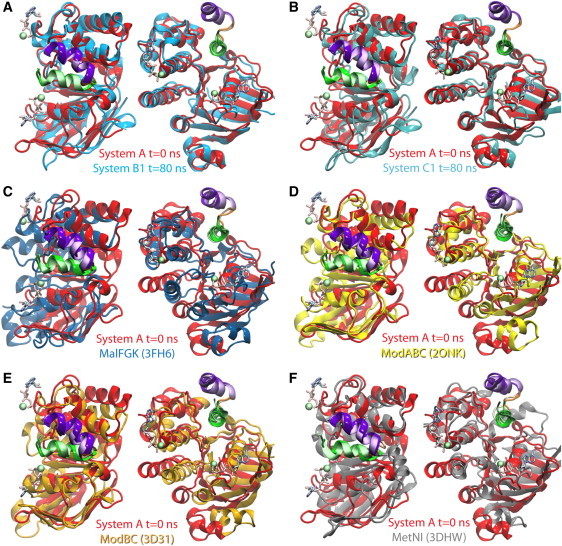
NBD-TMD coupling in small ABC importers. The starting structure of simulation System A (equivalent to the crystal structure 2R6G (8) with the coordinates of MgATP) is superimposed with end structures of simulation Systems B1 and C1, as well as with several other crystal structures of small ABC importers. The superposition is performed using the EAA loop (green and purple helices connected by an orange loop, colored as in Fig. 1), and the orientation of the flanking NBDs are compared. In the reference structure, labeled System A, the EAA loop is shown in a glossy representation and the NBD in red; in other structures the EAA loop is drawn using faded colors and the NBDs in various colors: (A) the end structure of System B1 in cyan; (B) the end structure of System C1 in teal; (C) the resting state crystal structure of the maltose transporter (PDB:3FH6 (12)) in dark blue; (D) the molybdate/tungstate transporter of Archaeoglobus fulgidus (PDB:2ONK (6)) in yellow; (E) the molybdate transporter of Methanosarcina acetivorans (PDB:3D31 (10)) in brown; (F) the methionine transporter of Escherichia coli (PDB:3DHW (11)) in gray. In each panel, the structures are shown both in top (extracellular) view (left), and in side view (right). MgATP is shown in System A as a point of reference to highlight the ATP-binding sites.
