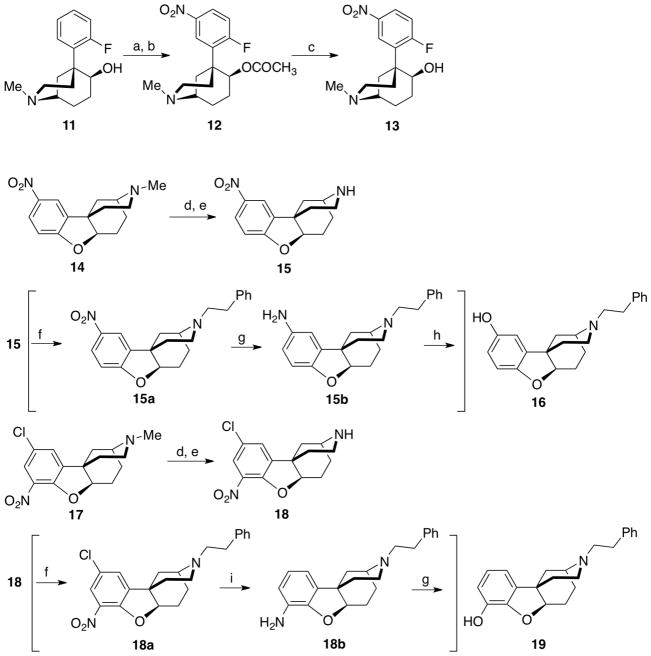
Scheme 2.
Improved steps in synthesis of ortho and para-b isomers. Reagents and conditions: (a) Ac2O, AcOH, 90 °C; (b) fuming HNO3, 92–96% from 11; (c) 2N HCl, reflux 3 h, 5N NaOH, 93–96%; (d) BrCN, K2CO3, acetonitrile, reflux 2 H; (e) HCl, AcOH, reflux 18 h; (f) Ph(CH2)2Br, NaI, CH3CN, reflux; (g) 10% Pd-C, EtOH; (h) NaNO2, Cu(NO3)2• 2.5H2O, Cu2O, 35% H2SO4; (i) 10% Pd-C, HCO2NH4, EtOH. For experimental procedures used to prepare 15a, 15b, 18a, and 18b, see Kurimura et al.4