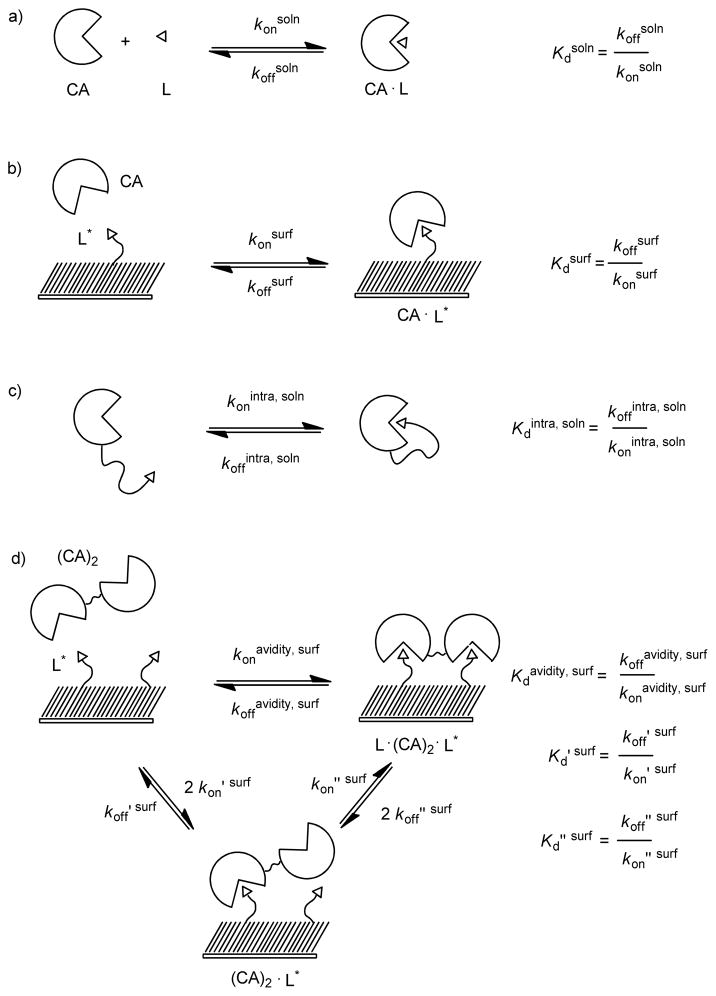
Scheme 1.
Thermodynamic schemes describing the binding of monovalent carbonic anhydrase CA and bivalent carbonic anhydrase (CA)2 to surfaces presenting benzensulfonamides (L). a) The binding of CA to a monovalent ligand in solution (L) forms a receptor-ligand complex (CA·L) that is characterized by rate constants for association and dissociation, konsoln and koffsoln, respectively. The ratio koffsoln to konsoln is the dissociation constant Kdsoln. b) The binding of CA to a mixed SAM presenting multiple ligands forms a receptor-ligand complex (CA·L*) that is characterized by rate constants for association and dissociation, konsurf and koffsurf. The ratio koffsurf to konsurf is the dissociation constant Kdsurf (eq 2). c) The rate of association and dissociation between the binding site of CA and a ligand covalently attached to CA by a flexible tether is characterized by rate constants for association and dissociation, konintra, soln and koffintra, soln, respectively. The ratio koffintra, soln to konintra, soln is the unitless dissociation constant Kdintra, soln. d) The association of (CA)2 to two ligands (L*) can be conceptualized as a process involving two steps. The initial step—the association of (CA)2 to L*—is characterized by rate constants koff ′ surf and kon′ surf. The second step—the association of an additional ligand to the unbound active site of (CA)2 forms a complex consisting of one (CA)2 and two ligands (L*· (CA)2·L)* —is characterized by rate constants koff ″ surf and kon ″ surf. The ratio koff ″ surf to kon ″ surf is the dissociation constant Kd″ surf (eq 6). The rate of binding of (CA)2 to two ligands is characterized by rate constants koffavidity, surf and konavidity, surf. The ratio of koffavidity, surf and konavidity, surf is the avidity (Kdavidity, surf) and characterizes the strength of (CA)2 binding to L* in the form of L*·(CA)2·L* (eq 8).