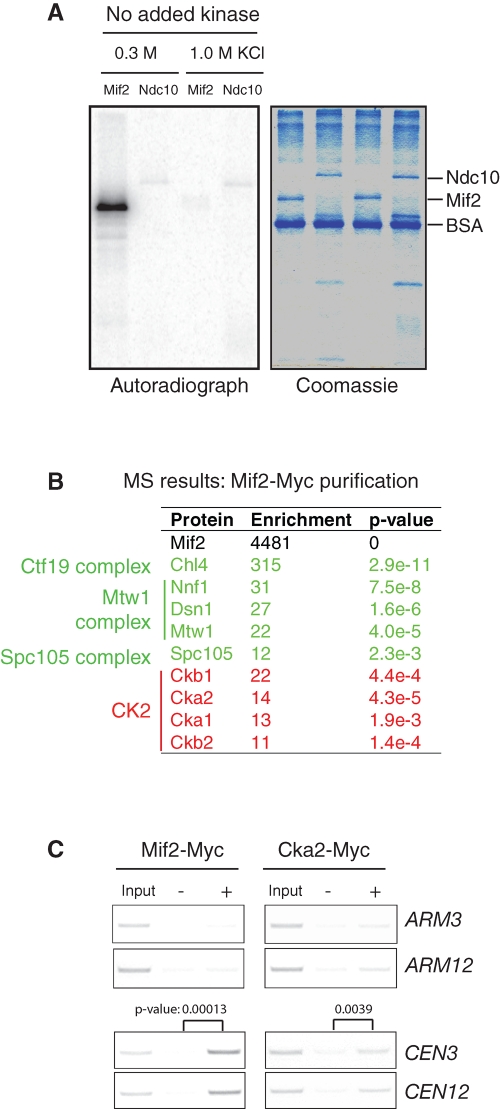
FIGURE 1:
CK2 associates with kinetochores. (A) A protein kinase activity copurifies with Mif2. Mif2-Myc and Ndc10-Myc were overexpressed using a GAL1 promoter in yeast and were affinity-purified using anti-Myc antibodies and a moderate (0.3 M KCl) or high (1 M KCl) salt concentration. The purified proteins were incubated with [γ32P]ATP for 30 min at 25°C. The samples were analyzed by autoradiography following SDS–PAGE. (B) MS identified all four subunits of CK2 as copurifying with Mif2. The peptide count for each protein was used to estimate its abundance. The enrichment of each protein is equal to a ratio of the peptide counts in the purified Mif2 sample to the total peptide counts (Peptide Atlas: www.peptideatlas.org). p Values for enrichment were calculated using Fisher's exact test. (C) Cka2, a catalytic subunit of CK2, associates with centromeric DNA. Cells expressing Mif2-Myc or Cka2-Myc at their endogenous loci were analyzed by ChIP. The centromere and arm of chromosome 3 and chromosome 12 were amplified by PCR from either the total input chromatin (Input), immunoprecipitated samples (+), or mock-treated (without antibody) controls (−). Three independent experiments were carried out, and one representative result is shown. For centromere binding, the results were quantified using ImageJ; p values were determined using Student's t test.