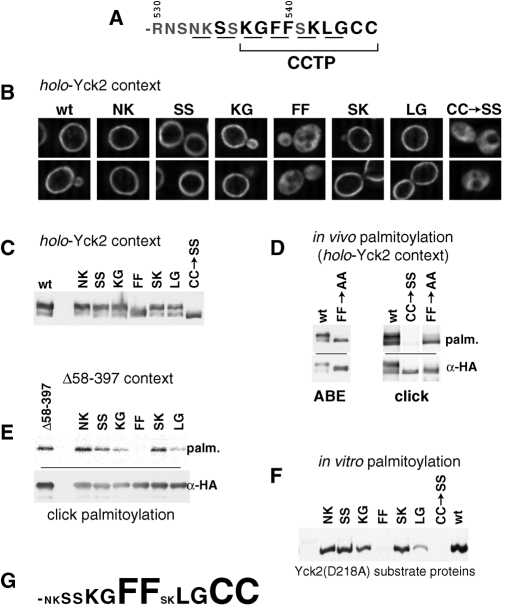
FIGURE 5:
Analysis of CCTP di-alanine substitution mutants. Consecutive residues across the C-terminal Yck2 14 amino acids were replaced with Ala-Ala. The di-alanine substitution mutations were compared with the unsubstituted, wt sequence and to the nonpalmitoylatable CC->SS replacement for effects on palmitoylation, phosphorylation, and localization within both the holo-Yck2 context as well as within the KD-deleted Yck2(Δ58-397) context. (A) Yck2 C-terminal sequence with the residues that were substituted by Ala-Ala are underlined. (B) Effects of di-alanine substitutions on localization assessed within the holo-Yck2 context by IIF microscopy. (C) Hyperphosphorylation of di-alanine substitution mutants assessed within the holo-Yck2 context by anti-HA Western blotting. (D) The effect of the FF->AA mutation on palmitoylation assessed within the holo-Yck2 context, using either the ABE or click-based detection methodologies (see Materials and Methods). (E) Palmitoylation effects of di-alanine mutations were tested within the partially impaired Yck2(Δ58-397) context using the click-based detection methodology (see Materials and Methods). (F) Palmitoylation effects of di-alanine mutations were tested in an in vitro system, using purified Akr1 and purified, di-alanine-substituted Yck2 substrate proteins (see Materials and Methods). Palmitoylation competence was assessed by the acceptance of [3H]-palmitate label donated from labeled palmitoyl-CoA. Note that the purified substrate proteins tested here all have the kinase-inactivating D218A mutation in addition to the mutations indicated. (G) Relative contribution of individual CCTP dipeptides.