Figure 8. Disruption of mGluR signalling abolishes synaptically induced endocannabinoid-mediated retrograde suppression of PF EPSCs in sg/sg PCs.
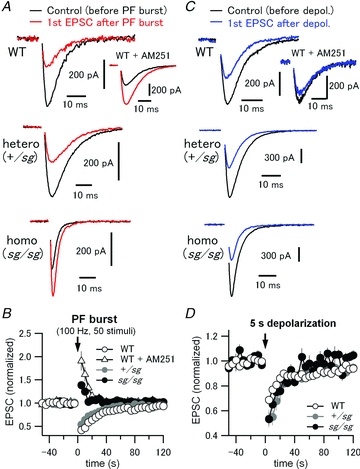
A, PF-evoked EPSCs were monitored in PCs every 5 s. After recording 10 stable basal EPSCs, a high frequency PF stimulus train (PF burst, 50 pulses at 100 Hz) was applied to activate an mGluR-mediated signalling cascade which causes endocannabinoid-dependent retrograde suppression of PF EPSCs for tens of seconds. After PF burst, 24 PF EPSCs were recorded to monitor such short-term plasticity, which is also known as synaptically evoked suppression of excitation (SSE). Black and red traces represent the last EPSC of the basal EPSC recordings (the EPSC just before PF burst) and the first EPSC after PF burst in each experimental condition, respectively. Inset in the top panel shows the traces obtained in the same way except from a different PC in the presence of a CB1-receptor antagonist, AM251 (5 μm). B, peak amplitudes of all the recorded EPSCs were normalized to averaged peak amplitudes of 6 basal EPSCs evoked before PF burst. Each data point represents mean normalized amplitude plotted against the recording time. An arrow indicates the time point zero when the PF burst was applied. C and D, depolarization-induced suppression of excitation (DSE), where endocannabinoid release from PCs depends solely on a large intracellular Ca2+ increase, but not on activation of mGluRs. Experimental protocol and data analysis were the same as in A and B, except that a 5 s depolarizing pulse to 0 mV was applied to PCs instead of PF burst for induction of DSE. In contrast to the SSE data in A and B, DSE was reliably observed in WT and staggerer mutant mice, indicating that endocannabinoid production and presynaptic CB1-receptor function are both intact in the mutants.
