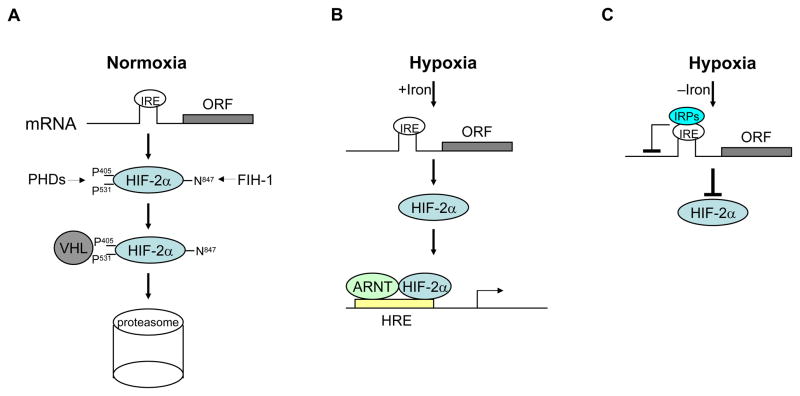
Figure 1. Regulation of HIF-2α expression.
A, Under normoxia, HIF-2α is hydroxylated by PHDs at proline 406 and 531. Hydroxylation leads to pVHL binding and subsequent degradation of HIF-2α by the 26S proteasome. B, Under hypoxia, when iron levels are adequate, HIF-2α is stabilized due to inhibition of PHD activities and forms complexes with ARNT to transactivate expression of hypoxia inducible genes. C, Under hypoxia, when iron is deficient, IRPs bind to the IRE within 5′UTR of HIF-2α mRNA, which in turn inhibits the translation of HIF-2α. See text for details. IRE: iron response element; IRP: IRE binding pritein; PHD: prolyl hydroxylase-domain protein; FIH: factor inhibiting HIF.