SUMMARY
In spite of the amount of literature demonstrating the relationship between upper and lower airways, both from the anatomical, and pathophysiological point of view, little is known about the epidemiology, diagnosis and treatment of the Rhino-Bronchial Syndrome (RBS). After the publication, in 2003, of a Consensus Report defining the Rhino-Bronchial Syndrome, an interdisciplinary group of experts made up from the Italian ENT Society (SIO) and the Interdisciplinary Scientific Association for the Study of Respiratory Diseases (AIMAR) met again in 2005 in order to study a protocol which would have, as the main tasks, the analysis of RBS signs and symptoms and standardization of the diagnostic approach. A secondary endpoint was to characterize the most effective therapeutic options and to correct the great dyshomogeneity in the therapeutic approaches. With this aim, 9 ENT and Pneumology Centres were selected, based on the ability to multidisciplinary cooperation, availability of useful instrumentation and homogeneous distribution over the entire National territory. Overall, 159 patients were enrolled according to clinical history (major and minor symptoms of upper and lower airways) and inclusion/exclusion criteria. All underwent a two level diagnostic approach. In 116 patients, the diagnosis was confirmed on the basis of I level (rhinopharyngeal endoscopy and basal spirometry, respectively, for upper and lower airways) examination. Allergic and infectious diseases were significantly more frequent (37.9% vs 20.9% and 73.3% vs 46.55, respectively) in patients with a confirmed diagnosis for Rhino-Bronchial Syndrome. Nasal obstruction (93%), rhinorrhoea (75%), cough (96%) and dyspnoea (69%) were the more frequent symptoms. The presence of meatal secretions or polyps were the clinical findings significantly differing at endoscopy in the two groups. After 3 months of treatment, according to "good clinical practice" (inhaled steroids, antibiotics, nasal lavages), 96% of the patients recovered. On the basis of these results, a diagnostic flow-chart is proposed according to which the persistence of some symptoms (cough, dyspnoea, rhinorrhoea and nasal obstruction) should lead the patient to a multidisciplinary and multi-level diagnostic approach by an otorhinolaryngology and a pneumology specialist working together for a definitive diagnosis. The recovery rate of about 94% of patients after 3 months of treatment, stresses the importance of a correct diagnosis.
KEY WORDS: Upper and lower airways, Rhino-Bronchial Syndrome, Inflammatory cytokines, Rhino-bronchial reflex, Diagnosis, Treatment
RIASSUNTO
Nonostante le numerose ricerche rinvenibili in letteratura a sostegno di una correlazione anatomica e fisiopatologia fra alte e basse vie aeree, poco si sa dell'epidemiologia, della diagnosi e del trattamento della Sindrome Rino-Bronchiale (SRB). Dopo la pubblicazione nel 2003 di una Consensus per la definizione della Sindrome Rino-Bronchiale, un gruppo multidisciplinare di esperti composto da rappresentanti della SIO (Società Italiana di Otorinolaringologia e Chirurgia Cervico-Facciale) e dell'AIMAR (Associazione Interdisciplinare per lo studio delle Malattie Respiratorie), si è riunito nuovamente nel 2005 con l'intento di mettere a punto un protocollo di studio avente come obiettivi principali l'analisi dei segni e sintomi della Sindrome Rino-Bronchiale e la standardizzazione dell'approccio diagnostico. Obiettivo secondario del protocollo era l'individuazione delle opzioni terapeutiche più efficaci per correggere l'elevata disomogeneità a tutt'oggi presente nel trattamento della sindrome. In quest'ottica sono stati selezionati 9 centri distribuiti su tutto il territorio nazionale che avessero la possibilità di una collaborazione interdisciplinare e fossero forniti di necessaria strumentazione. Sulla base della storia clinica (segni e sintomi maggiori e minori a carico delle vie aeree superiori ed inferiori) e dei criteri di inclusione ed esclusione, sono stati arruolati 159 pazienti. In 116, la diagnosi di Sindrome Rino-Bronchiale è stata confermata sulla base di un protocollo diagnostico che prevedeva indagini di I (endoscopia rinofaringea e spirometria basale rispettivamente per le vie aeree superiori ed inferiori) e II livello. Le patologie infiammatorie ed infettive erano significativamente più frequenti nei soggetti con diagnosi di Sindrome Rino-Bronchiale confermata (37,9% vs 20,9% e 73,3% vs 46,55% rispettivamente). L'ostruzione nasale (93%), la rinorrea (75%), la tosse (96%) e la dispnea (69%) sono stati i sintomi più frequentemente rilevati. La presenza di secrezioni in corrispondenza dell'ostio-meato e/o di polipi all'indagine endoscopica differiva significativamente nei due gruppi (diagnosi confermata o no). Al termine di 3 mesi di trattamento seguendo i criteri della "buona pratica clinica" (cortisonici per aerosol, antibiotici, lavande nasali) la risoluzione della sintomatologia è stata rilevata nel 96% dei pazienti. Sulla base dei risultati gli Autori propongono una flow-chart diagnostica che preveda un approccio integrato e multilivello tra specialisti delle vie aeree superiori ed inferiori in caso di presenza e persistenza di tosse, dispnea, ostruzione nasale, rinorrea. La risoluzione della sintomatologia nel 94% dei pazienti dopo tre mesi di terapia, sottolinea l'importanza di un corretto approccio diagnostico quale premessa alla terapia.
Introduction
Various studies have demonstrated that the upper and lower airways have not only an anatomical continuity, but adopt, also, the same physiopathological mechanisms in order to answer pathogenic noxae; therefore they must be considered as a unique morphofunctional unit.
The key pathogenic mechanism is the inflammation of the upper airways no matter how it was induced, which is able to extend to the lower airways inducing also a systemic deregulation, with a complex interaction between cells and inflammatory cytokines.
But, if it is, by now, true that a lot that we know with regard to the pathogenetic mechanisms that relate upper and lower airways 1-7, little, on the contrary, is known about the epidemiology, the diagnostic approach and the therapeutic behaviour to be adopted in clinical pictures involving these two districts.
In particular, as far as concerns the pathogenesis, rhinobronchial reflex 8 9 is one of the mechanisms able to explain the upper-lower airways relationship, even if the exact anatomical location of the afferent branch of this reflex has not yet been clearly defined. Namely, application, on the nasal mucosa, of a stimulus such an allergen or (in some cases) just cold air, is able to determine an immediate broncho-spasm. Moreover, we must remember the role of descending infections (post‑nasal drip): falling of inflammatory material (mucous-pus and inflammatory cells) from the upper airways towards the lower district, inhaled because of the inspiratory activity of the lungs, could represent an important irritating trigger 10 11.
Last, but not least, a third extremely important direct pathogenetic mechanism is represented by the diffuse mucosal inflammation, caused by activation of eosinophils. According to this hypothesis, any inflammation in the upper airways (independently of the origin: allergic, infective or irritative) is a fundamental prodromic event for the extension of the pathology to the lower airways.
Specifically, eosinophils, through the release of specific mediators, such as ECP (Eosinophil Cationic Protein), EPO (Eosinophil Peroxidase), MPO (Myeloperoxidase) or NMP (Nitrate-Methyl-Proxyl), potent NO-donor (nitric oxide-donor) involved in all the oxidative stress-mediated pathologies, seem to be able to induce diffuse mucosal damage throughout the entire respiratory tree 12.
The Consensus Report on the Rhino-Bronchial Syndrome, processed in 2003 by the interdisciplinary group of the Italian Otorhinolaryngology Society (SIO) and the Interdisciplinary Association for the Study of the Respiratory Diseases (AIMAR) defined the Rhino-Bronchial Syndrome (RBS) as "a nosologic entity which develops when a hyper-reactive process or a recurrent or chronic inflammation of the upper airways, or anatomical alterations of the rhinosinusal district, facilitate the development of an inflammatory state, on an infectious or immunological basis, in the lower airways, compromising also their function" 13.
In this context, a Scientific Committee, made up of experts from the two Societies, has attempted to define a study protocol which had, as the main task, to analyze signs and symptoms of RBS and to standardize the diagnostic approach, defining the first and second level examinations to be performed.
The secondary endpoint of the study was to characterize, between the various therapeutic options, those most effective, thus contributing, to correct the great dishomogeneity, in the therapeutic approaches, currently proposed.
Material and methods
In 2005, 9 ENT and Pneumology Centres were identified (Fig. 1) and selected, based on the following requirements: ability to multidisciplinary cooperation, availability of useful instrumentations for the study and homogenous distribution over the entire National territory.
Fig. 1.
ENT and Pneumology centres involved in the study protocol on Rhino- Bronchial-Syndrome.
After a meeting of investigators to define the sharing of the protocol and after submission of the same for approval of the Ethics Committee of the Coordinating Centre, it was agreed, starting from 1.1.2006, to start the enrollment of all the subjects who, in the course of the year consecutively, referred to the outpatient departments (every patient had to be estimated by both the specialists) and who met the following inclusion criteria:
males and females aged between 18 and 70 years;
-
typical symptoms:
presence of at least one major criterion for both upper and lower airways;
presence of one major criterion for lower airways and 2 minor criteria for upper airways.
Major Criteria: U pper airways: nasal obstruction, postnasal drip, cough. Lower airways: cough, dyspnoea, sputum.
Minor Criteria: Rhinorrhoea, itching, anosmia, sore throat, facial pain, nose bleeding, fever.
Exclusion criteria were:
patients submitted, in the last 3 months, to upper or lower airways surgical procedures;
patients with active oncologic conditions;
patients with heart failure (NYHA class II or above);
patients taking ACE-inhibitors;
recent or ongoing pneumonia (2 months);
patients with TBC;
immunocompromised patients;
HIV patients;
pregnant women;
patients with genetic disorders.
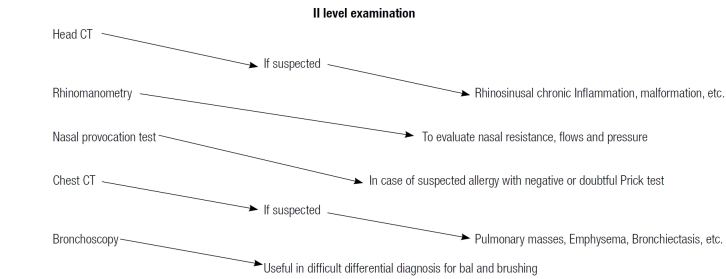
All clinical data, collected in the various centres, were stored on an on-line database for the statistical analysis. Each patient, enrolled according to clinical history and inclusion criteria, underwent a two-level diagnostic apapproach, as shown in Table I and II. All the investigations were performed within 4 months from the enrollment.
Table I.
Diagnostic approach.
| I level examination | |
|---|---|
| Clinical history | |
| Infectious form suspected | Immunologic form suspected |
| ENT & pneumologist | ENT & pneumologist |
| Upper airway endoscopy | Upper airway endoscopy |
| Respiratory functionality | Respiratory functionality |
| Sputum bacteriology | Sputum bacteriology |
| Mucociliary transport time | Prick Test |
| Neutrophils count in nasal secretion | Mucociliary transport time |
| Chest X-ray | Eosinophils count in nasal secretion |
Table II.
Diagnostic approach.
 |
After enrollment of patients, the clinical diagnosis was confirmed, or excluded, according to the examinations defined by the "2003 SIO-AIMAR Consensus report" and the patients were treated according to the "good clinical practice" by the enrolling specialists. Specifically, concerning upper airway disorders, when chronic rhinosinusitis was diagnosed, on the basis of symptoms (nasal obstruction, secretion) and signs (mucosal hypertrophy, secretion at the ostio-meatal complex) by nasal endoscopy and/or CT scan, topical steroids (mometasone furoate, fluticasone proprionate and fluticasone furoate) were used on alternate months for 3 months (200 or 400 μg/ day according to the severity of the clinical picture). This treatment regimen was not changed when nasal polyps, confined to the middle meatus were present. Nasal lavages/ douching with isotonic solution were suggested, once or more times/day, to all the patients. Oral anti-histamines or leukotrienes antagonist were prescribed only in the case of allergy confirmed by II level investigations (prick test, nasal provocation test). Oral corticosteroids (methylprednisolone, prednisone) were used, only seldomly, as adjunctive therapy to antibiotics (amoxicillin-clavulanic acid, cefuroxime axetil, levofloxacin) only in cases of acute episodes of rhinosinusitis with severe symptoms (facial pain, headache).
As far as concerns lower airway disorders, when obstruction was diagnosed on the basis of symptoms (dyspnoae, secretion, cough) and functional tests (spirometry, methacholine test, Beta2 test), oral corticosteroids and/or aerosol treatment with corticosteroid, beta2 adrenergic and anti-cholinergic drugs were used; antibiotics and mucolytic agents were used only when an infectious disease was diagnosed.
All the subjects were re-evaluated 3 months after enrollment.
A statistical analysis was performed using SPSS software; the significance index was evaluated using the χ2 Test.
Results
A total of 230 patients were recruited, of whom, only 159 were considered valid for statistical analysis. Of these, 116 had a confirmed diagnosis of RBS, according to the "2003 SIO-AIMAR Consensus report". The 43 subjects, in whom the RBS diagnosis was not confirmed, were used as a control group.
Both allergic and infectious respiratory disorders were more frequent in subjects with a confirmed diagnosis (Table III ). Specifically, an allergic sensitization was recorded in 37.9% of patients with a confirmed diagnosis of RBS compared with 20.9% in whom the diagnosis was not confirmed (p < 0.05). Moreover, the percentage of rhinosinusal infections, in the two groups, was: 73.3% (RBS confirmed) and 46.5% (RBS not confirmed) (p = 0.002). Bronchial infections reached 69.8% when the RBS diagnosis was confirmed, compared with 34.9% of patients in whom diagnosis was not confirmed (p = 0.001).
Table III.
Population data and clinical history.
| Population Data | RBS confirmed (n = 116) |
RBS not confirmed (n = 43) |
p |
|---|---|---|---|
| Age (yrs) (mean ± SD) | 48.9 ± 13.1 | 45.5 ± 14.9 | |
| Sex no. (%) | |||
| M | 55 (47.4) | 29 (67.4) | 0.025 |
| F | 61 (52.6) | 14 (32.6) | |
| Clinical history | |||
| Smoker no. (%) | |||
| YES | 32 (27.6) | 10 (23.3) | 0.065 |
| NO | 57 (49.1) | 15 (34.9) | |
| Ex | 27 (23.3) | 18 (41.9) | |
| No. Sig./die (mean ± DS) | 17.5 ± 10.6 | 10.4 ± 5.7 | 0.049 |
| Alcohol no. (%) | |||
| YES | 63 (54.3) | 27 (62.8) | 0.338 |
| NO | 53 (45.7) | 16 (37.2) | |
| Allergy no. (%) | |||
| YES | 44 (37.9) | 9 (20.9) | 0.04 |
| NO | 72 (62.1) | 34 (79.1) | |
| Respiratory infections no. (%) | |||
| No infection | 13 (11.2) | 7 (16.3) | |
| Rhinosinusitis | 85 (73.3) | 20 (46.5) | 0.002 |
| Pharyngitis | 76 (65.5) | 16 (37.2) | 0.000 |
| Bronchitis | 81 (69.8) | 15 (34.9) | 0.001 |
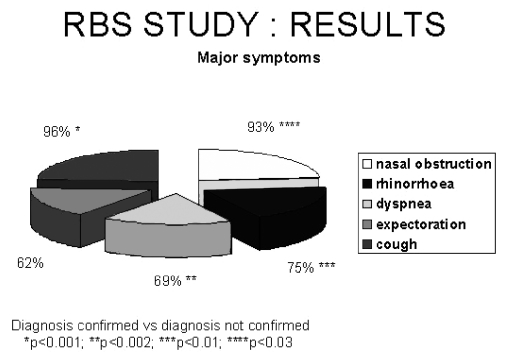
The distribution of major symptoms is shown in Figure 2; as can be seen, some symptoms were more frequent in patients with confirmed RBS and specifically: nasal obstruction (93%, p < 0.03) rhinorrhoea (75%, p < 0.01), cough (96%, p < 0.001) and dyspnoea (69%, p < 0.002).
Fig. 2.
Distribution of major symptoms in patients with confirmed diagnosis.
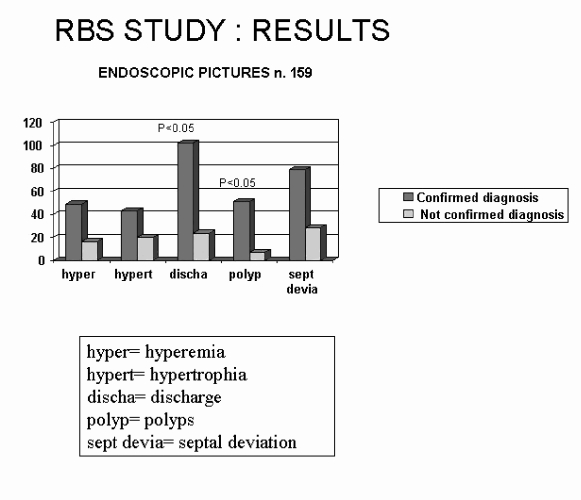
Regarding ENT instrumental examinations, all subjects underwent flexible (or rigid) rhino-endoscopy. Endoscopic pictures are shown in Figure 3: briefly, the presence of meatal secretions (p < 0.05) and polyps (p < 0.05) were the clinical findings significantly differing in the two groups.
Fig. 3.
Endoscopic findings in 159 patients with confirmed/not confirmed RBS diagnosis.
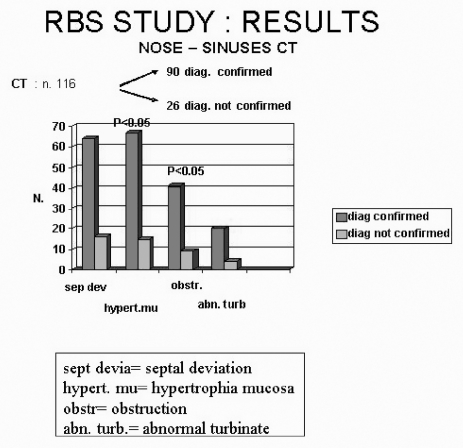
Concerning II level examinations, imaging studies of the upper airways were frequently performed; namely, 116 computed tomography (CT) skull-facial scans were performed. At CT scan analysis, mucosal hypertrophy and complete obstruction of the ostio-meatal complex were more frequent in confirmed RBS patients than in the control group (p < 0.05) (Fig. 4). Other II level ENT tests, such as nasal decongestion test, mucociliary transport time, rhinomanometry were performed only in a few cases with no significant effects on diagnostic accuracy.
Fig. 4.
CT findings in patients with confirmed diagnosis and in control group (diagnosis not confirmed).
Focusing on pneumological investigations, all the patients underwent basal spirometry, whereas a bronchoreversibility test with salbutamol was performed in 44 patients and the hyper-responsiveness test with methacholine in 12 patients; in a large number of patients, the diagnostic evaluation was completed with chest X-ray (120 patients) or CT scan (27 patients) (Table IV).
Table IV.
Pneumologic tests.
| RBS confirmed (n. 116) |
RBS not confirmed (n. 43) |
|
|---|---|---|
| FEV1/FVC | ||
| Obstruction | 19 (17.6) | 1 (2.4) |
| Normal | 89 (82.4) | 40 (97.6) |
| FEV1pc | ||
| Mild obstruction | 83 (74.1) | 38 (95.0) |
| Moderate obstruction | 26 (23.2) | 2 (5.0) |
| Severe obstruction | 3 (2.7) | – |
| Bronchoreversibility Test | 31 (26.7) | 13 (30.2) |
| Positive | 12 (38.7) | 1 (7.7) |
| Negative | 19 (61.3) | 12 (92.3) |
| Bronchostimulation | 11 (9.5) | 1 (2.3) |
| Positive | 11 (100) | – |
| Negative | – | 1 (100) |
| Chest X-ray | 86 (74.1) | 34 (79.1) |
| Positive | 18 (20.9) | – |
| Negative | 68 (79.1) | 34 (100) |
| Thorax CT | 23 (19.8) | 4 (9.3) |
| Positive | 15 (65.2) | 1 (25.0) |
| Negative | 8 (34.8) | 3 (75.0) |
| Prick- test | 40 (34.5) | 13 (30.2) |
| Monosensitization | 12 (10.3) | 3 (7.0) |
| Polysensitization | 14 (12.1) | 2 (4.7) |
| Negative | 14 (12.1) | 8 (18.5) |
| Not performed | 76 (65.5) | 30 (69.8) |
In 116 patients with an anatomical or inflammatory alteration or disease of the upper airways, pneumologists highlighted the following concomitant pathological conditions: chronic bronchitis (73 patients), obstructive chronic bronchitis (19 patients), asthma (43 patients), bronchiectasis (11 patients); in some of them, two or more of the abovementioned lower airways disorders coexisted.
The different treatment options selected by the various specialists involved in the study are reported in Table V . The most commonly administered drugs were inhaled steroids, either as bronchial or nasal (44%), antibiotics (42%), and nasal lavages (25.9%).
Table V.
Treatment in 116 patients with confirmed diagnosis of RBS.
| Treatment | Number (%) |
|---|---|
| Nasal steroids | 52 (44.8) |
| Antibiotic | 49 (42.2) |
| Inhalatory steroids | 35 (30.2) |
| Nasal lavages | 30 (25.9) |
| Mucolitics | 22 (19.0) |
| Systemic steroids | 16 (13.8) |
| Beta2 stimulating | 15 (12.9) |
| Anti-histamines | 9 (7.8) |
| Anti-leukotrienes | 7 (6.0) |
| No treatment | 10 (8.6) |
After 3 months of treatment, 73% of the sample (85 patients) was re-evaluated. In 94% of whom a significant improvement in the clinical picture was recorded, whereas 6% of the patients remained unchanged.
Discussion
Over the years, the scientific contributions revealing the association between rhinosinusal disorders and lower airways diseases have definitively confirmed the interrelation of the two districts and redefined the causative pathogenetic mechanisms.
Concerning the rhinitis-asthma relationship, the reports from Braunstahl et al., analyzed, after nasal provocation with an allergen, the degree of infiltration of mucosal eosinophils in a group of non-asthmatic subjects, affected by allergic rhinitis, compared to a control group; they showed an increased and significant infiltration of eosinophils in both the nasal and bronchial mucosa, independently of the district (nose or lung) exposed to the allergenic stimulation 14 15. Similar results were obtained in studies focusing on the vascular expression of ICAM-1 16.
A report showing a correlation between COPD (Chronic Obstructive Pulmonary Disease) and upper airways inflammation was presented by Hurst et al., in 2005; they evaluated nasal and bronchial inflammation in patients with COPD, by estimating the IL-8 concentration (a cytokine with a wellknown chemotactic and activating effect on neutrophils), in comparison with a control group 17. A review on scientific evidence supporting the link between non-allergic upper airways disorders and asthma was made by Corren et al. 18: according to several studies most patients with non-allergic asthma have chronic nasal symptoms as well as radiographic evidence of sinus mucosal disease. Equally important, preexisting symptoms of rhinitis make non-allergic patients at higher risk of developing asthma. Systemic circulation plays a key role in amplifying inflammation in other portions of the respiratory tract.
According to Corren et al. 18, this significant body of literature on nose-lung correlations and the complex relationship between localized and systemic inflammation, indicate that it is necessary to assess and treat rhinitis and sinusitis when they are present in patients with asthma. Common diagnostic criteria and treatment protocols shared among a wide variety of practitioners, including otorhinolaryngologists, pulmonologists, primary care physicians and allergologists will lead to a reduction in lower airways hyperesponsiveness, improving the patients' quality of life 19.
Our results show, first of all, that chronic or recurrent upper airways infections (rhinosinusitis, pharyngitis) have a double prevalence in patients affected by RBS, that is why it is extremely important for the lower airways specialist, to investigate the clinical history of upper airways for a valid diagnostic approach.
Some prevalent symptoms, such as nasal obstruction, rhinorrhoea, dyspnoea, cough, must rise the suspicion of the entire respiratory tract involvement in the inflammatory process; they are, in fact, present in > 73% of patients with confirmed diagnosis of rhinobronchial syndrome.
Concerning the diagnostic approach, it is quite simple and can be standardized by the ENT specialist, as the gold standard test is flexible endoscopy of the rhinosinusal district; the CT scan of this region, although not essential for the diagnosis, offers some interesting clinical data. A very recent study confirms the correlation between endoscopic scores and COPD severity 19.
Other II level ENT tests, such as rhinomanometry and the nasal decongestion test, offer a good positive predictive value but are not mandatory for diagnosis. On the contrary, the pneumological evaluation is more complicated as clinical data, pulmonary functionality tests and imaging have to be integrated to make a correct diagnosis.
Interestingly, according to our results, as soon as RBS is correctly diagnosed, the therapeutic approach is not a problem, as revealed by a 3 months recovery rate of about 94% of the sample.
In conclusion, we propose a diagnostic flow-chart in which some symptoms, such as cough, dyspnoea, rhinorrhoea and nasal obstruction, if not completely recovered after the initial treatment by the general practitioner, should lead the patient to seek an ENT and pneumological evaluation for a definitive diagnosis and treatment. In turn, these two specialists have to cooperate in order to guarantee a multidisciplinary and multi-level diagnostic approach for any patient with a rhinobronchial syndrome not yet confirmed.
In particular, the ENT specialist, after having carefully evaluated the upper airways by means of fiberoptic endoscopy, should investigate, in these patients, the possibility of recurrent bronchitis or other lower airways-related problems and where necessary, referring the patient for pneumological evaluation. Likewise, the pneumologist should refer, to the ENT colleague, any asthmatic or COPD patient with a positive clinical history for rhinosinusitis or pharyngitis or complaining of chronic nasal obstruction (Fig. 5).
Fig. 5.
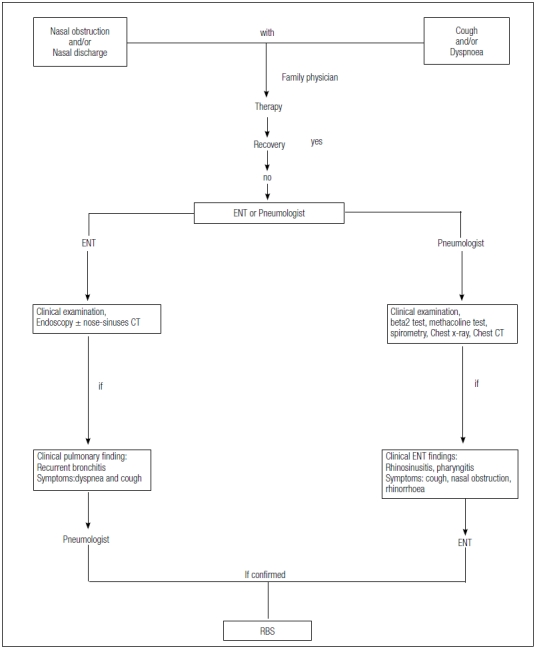
Integrated multidisciplinary diagnostic flow chart.
References
- 1.Harlin SR, Ansel DG, Lane SR. A clinical and pathologic study of chronic sinusitis: the role of the eosinophil. J Allergy Clin Immunol. 1988;81:867–875. doi: 10.1016/0091-6749(88)90944-x. [DOI] [PubMed] [Google Scholar]
- 2.Baroody FM, Hughes CA, McDowell P. Eosinophilia in chronic childhood sinusitis. Arch Otolaryngol Head Neck Surg. 1995;121:1396–1402. doi: 10.1001/archotol.1995.01890120054010. [DOI] [PubMed] [Google Scholar]
- 3.Benedictis FM, Bush A. Rhinosinusitis and asthma: epiphenomenon or causal association? Chest. 1999;115:550–556. doi: 10.1378/chest.115.2.550. [DOI] [PubMed] [Google Scholar]
- 4.Vinuya RZ. Upper airway disorders and asthma: a syndrome of airways inflammation. Ann Allergy Asthma Immunol. 2002;88:15–18. doi: 10.1016/s1081-1206(10)62023-6. [DOI] [PubMed] [Google Scholar]
- 5.Bachert C, Patou J, Cauwenberge P. The role of sinus disease in asthma. Curr Opin Allergy Clin Immunol. 2006;6:29–36. doi: 10.1097/01.all.0000200504.54425.0e. [DOI] [PubMed] [Google Scholar]
- 6.Shaaban R, Zureik M, Soussan D, et al. Allergic rhinitis and onset of bronchial hyperresponsiveness. Am J Resp Crit Care Med. 2007;176:659–666. doi: 10.1164/rccm.200703-427OC. [DOI] [PubMed] [Google Scholar]
- 7.Kim JS, Rubin BK. Nasal and sinus involvement in chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2008;14:101–104. doi: 10.1097/MCP.0b013e3282f4efc9. [DOI] [PubMed] [Google Scholar]
- 8.Barlìnski J, Gawin A, Doboszynska A. Naso-bronchial reflex in patients with allergic rhinitis. Pol Tyg Lek. 1988;43:407–408. [PubMed] [Google Scholar]
- 9.Kim JS, Rubin BK. Nasal and sinus inflammation in chronic obstructive pulmonary disease. COPD. 2007;4:163–166. doi: 10.1080/15412550701341228. [DOI] [PubMed] [Google Scholar]
- 10.Aubier M, Levy J, Clerici C, et al. Different effects of nasal and bronchial glucocorticosteroid administration on bronchila hyperresponsiveness in patients with allergic rhinitis. Am Rev Respir Dis. 1992;146:122–126. doi: 10.1164/ajrccm/146.1.122. [DOI] [PubMed] [Google Scholar]
- 11.Sanu A, Eccles R. Postnasal drip syndrome. Two hundred years of controversy between UK and USA. Rhinology. 2008;46:348–348. Author reply 348. [PubMed] [Google Scholar]
- 12.Braunstahl GJ, Fokkens W. Nasal involvement in allergic asthma. Allergy. 2003;58:1235–1243. doi: 10.1046/j.0105-4538.2003.00354.x. [DOI] [PubMed] [Google Scholar]
- 13.Benedetto M, Bellussi L, Cassano P, et al. Consensus Report on the diagnosis of rhino-bronchial syndrome (RBS) Acta Otorhinolaryngol Ital. 2003;23:406–408. [PubMed] [Google Scholar]
- 14.Braunstahl GJ, Overbeej SE, Kleinjan A, et al. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001;107:469–476. doi: 10.1067/mai.2001.113046. [DOI] [PubMed] [Google Scholar]
- 15.Braunsthahl GJ, Hellings PW. Nasobronchial interaction mechanisms in allergic airways disease. Curr Opin Otolaryngol Head Neck Surg. 2006;14:176–182. doi: 10.1097/01.moo.0000193186.15440.39. [DOI] [PubMed] [Google Scholar]
- 16.Chanez P, Vignola AM, Vic P, et al. Comparison between nasal and bronchial inflammation in asthmatic and control subjects. Am J Resp Crit Care Med. 1999;159:588–595. doi: 10.1164/ajrccm.159.2.9801022. [DOI] [PubMed] [Google Scholar]
- 17.Hurst JR, Wilkinson T, Perera WR, et al. Relationships among bacteria, upper airway, lower airway, and systemic inflammation in COPD. Chest. 2005;127:1219–1226. doi: 10.1378/chest.127.4.1219. [DOI] [PubMed] [Google Scholar]
- 18.Corren J, Kachru R. Relationship between nonallergic upper airway disease and asthma. Clin Allergy Immunol. 2007;19:101–114. [PubMed] [Google Scholar]
- 19.Piotrowska VM, Piotrowski WJ, Kurmanowska Z, et al. Rhinosinusitis in COPD: symptoms, mucosal changes, nasal lavage cells and eicosanoids. Int J Chron Obstruct Pulmon Dis. 2010;5:107–117. doi: 10.2147/copd.s8862. [DOI] [PMC free article] [PubMed] [Google Scholar]