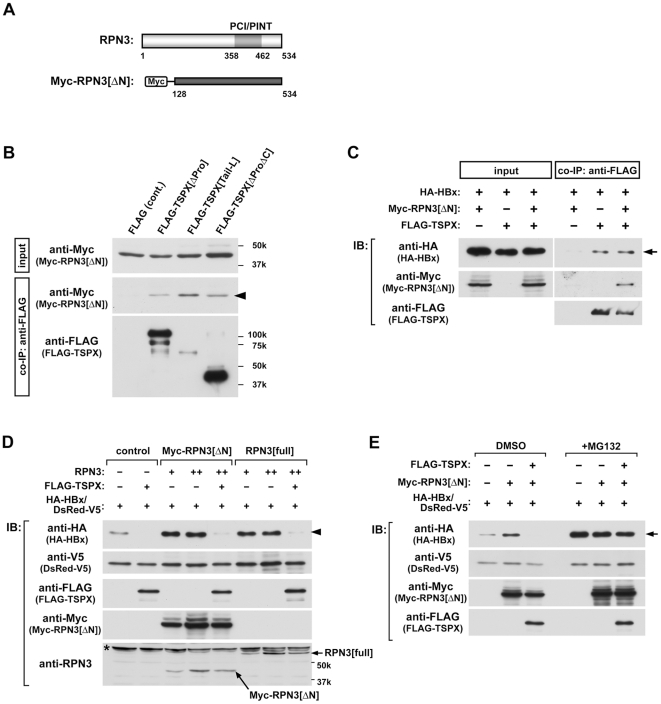
Figure 4. TSPX interacts with RPN3, and abrogates the protective effect of RPN3 on HBx-degradation.
(A) Structure of RPN3 and schematic representation of the truncated mutant of RPN3. The position of PCI/PINT domain is as marked. N-terminal truncated RPN3 (residues 128–534 a.a., termed as RPN3[ΔN]) was cloned into pCMV-Myc vector to express a Myc epitope-tagged product. (B) Interaction of TSPX and RPN3 in mammalian cells. Myc-RPN3[ΔN] was co-transfected with p3×FLAG-CMV7 (FLAG control), FLAG-TSPX[ΔPro], FLAG-TSPX[Tail-L] or FLAG-TSPX[ΔProΔC] into 293T cells. Immunoprecipitations were carried out as before using anti-FLAG antibody. Immunoblots were probed with anti-Myc and anti-FLAG antibodies. The immunoblots indicate that human RPN3 co-immunoprecipitates with human TSPX. (C) RPN3 did not interfere the interaction between TSPX and HBx. HA-HBx and FLAG-TSPX[ΔPro] expression vectors were co-transfected with or without Myc-RPN3[ΔN] vector into 293T cells. Immunoprecipitations were carried out using anti-FLAG antibody. Immunoblots were probed with anti-HA, anti-Myc and anti-FLAG antibodies, respectively. The results indicate that interaction between HA-HBx and FLAG-TSPX was not affected by co-expression of Myc-RPN3[ΔN]. (D) Over-expression of RPN3 protected HBx from protein-degradation, and TSPX overcomes the protective effect of RPN3. 293T cells were co-transfected with HA-HBx (0.2 µg/well), Myc-RPN[ΔN] (0.05, 0.1 µg/well), RPN3[full] (0.05, 0.1 µg/well) and/or FLAG-TSPX[ΔPro] (0.05 µg/well) as indicated in the figure. pcDNA-DsRed-V5 expression vector (0.1 µg/well) was co-transfected as the internal control for monitoring the transfection efficiency. Cells were lysed 48 h after transfection, and analyzed by Western blot using indicated antibodies. Although co-transfection of RPN3[full] or RPN3[ΔN] resulted in the increase of HA-HBx, TSPX significantly decreased the level of HBx even in the presence of RPN3. (E) Over-expression of Myc-RPN3[ΔN] did not affect on the transcription of HA-HBx. 293T cells were co-transfected with HA-HBx (0.2 µg/well), Myc-RPN[ΔN] (0.1 µg/well), and/or FLAG-TSPX[ΔPro] (0.05 µg/well) as indicated in figure. pcDNA-DsRed-V5 (0.1 µg/well) was co-transfected as the internal control for monitoring the transfection efficiency. Twenty-four hours after transfection, cells were treated with either vehicle (DMSO) or 25 µM MG132 for additional 24 h. Cells were lysed and analyzed by Western blot using anti-HA, anti-V5, anti-Myc, and anti-FLAG antibodies. Co-transfection of Myc-RPN3[ΔN] increased HA-HBx (DMSO). No significant difference was observed in HA-HBx level in the cells treated with MG132 (+MG132).