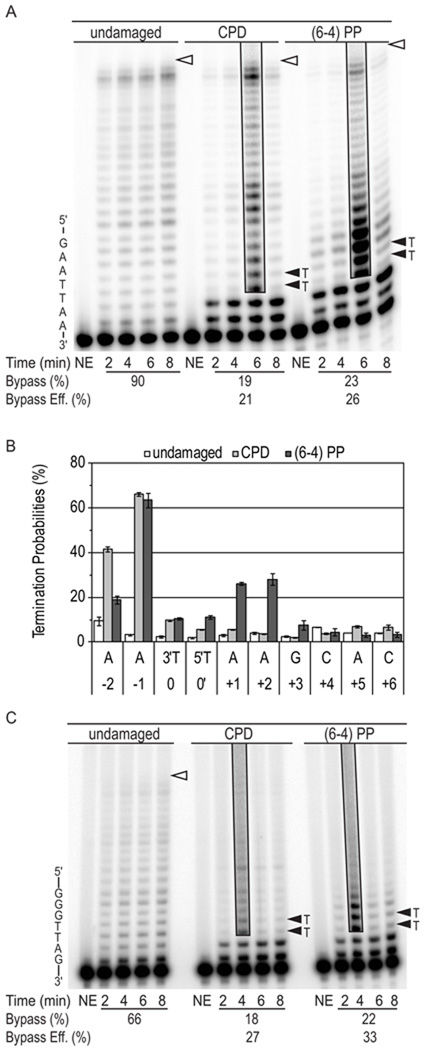
Figure 2. Bypass of UV photoproducts by L979F Pol ζ.
(A) Image of denaturing polyacrylamide gels showing products of synthesis by L979F Pol ζ using undamaged, CPD and (6-4) photoproduct (PP) 49-mer substrates. Primer extension reactions were performed in the presence of high dNTP concentrations intended to simulate damage-induced dNTP concentrations. Each reaction contained an 8-fold excess of substrate over polymerase. Part of the template sequence is shown on the left. The position of the 3′ and 5′ T of the CPDs and (6-4) PPs are indicated by closed arrowheads. Open arrowheads indicate the positions of full-length reaction products. Boxed areas of the gel show reaction products at an overexposed level of contrast to more clearly reveal less abundant DNA products of lesion bypass. NE indicates control lanes for reaction mixtures to which no enzyme was added. The average bypass probability for 2, 4, 6 and 8 min time points of each reaction and the relative bypass efficiency (bypass of damaged substrate relative to bypass of undamaged substrate) are shown under each gel. Duplicate reactions were performed with similar results. (B) Graph showing average probability of termination of processive synthesis at template nucleotides for 49-mer substrates, with error bars indicating standard deviation. (C) Image of denaturing polyacrylamide gels showing products of synthesis by L979F Pol ζ using undamaged, CPD and (6-4) PP 45-mer substrates.