Table 1.
RRRM through catalytic enantioselective silylation
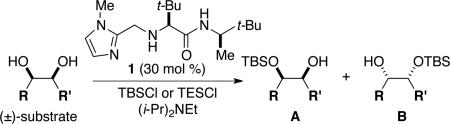 | |||||
|---|---|---|---|---|---|
| entry | substrate | A (ee) | B (ee) | yield (comb.) | ratio A/B |
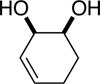
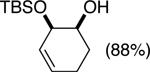
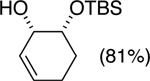
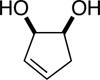
| (1) |
|
|
|
92%a | 50/50 |
| (2) |
|
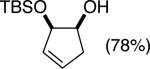
|
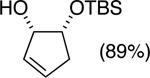
|
89%b | 58/42 |
| (3) |
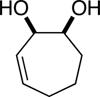
|
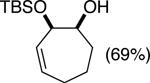
|
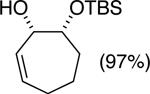
|
89%c | 64/36 |
| (4) |
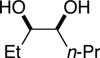
|
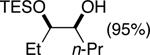
|
|
90%d | 50/50 |
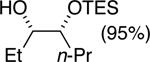
| (5) |
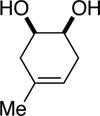
|
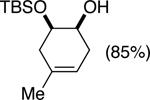
|
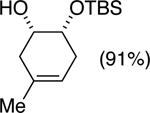
|
75%e | 52/48 |
| (6) |
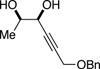
|
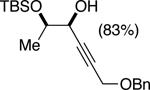
|
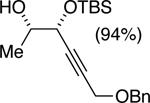
|
50%f | 52/48 |
Conditions:
TBSCl (2 equiv), DIPEA (1 equiv), toluene [1 M], -30 °C, 120 h.
TBSCl (1.5 equiv), DIPEA (1 equiv), toluene [1 M], -60 °C, 120 h.
TBSCl (1.5 equiv), DIPEA (1 equiv), toluene [1 M], -30 °C, 120 h.
TESCl (1.5 equiv), DIPEA (1 equiv), THF [1 M], -60 °C, 72 h.
TBSCl (1 equiv), DIPEA (1 equiv), toluene [1 M], -60 °C, 120 h.
TBSCl (1 equiv), DIPEA (1.25 equiv), THF [1 M], -30 °C, 110 h.
