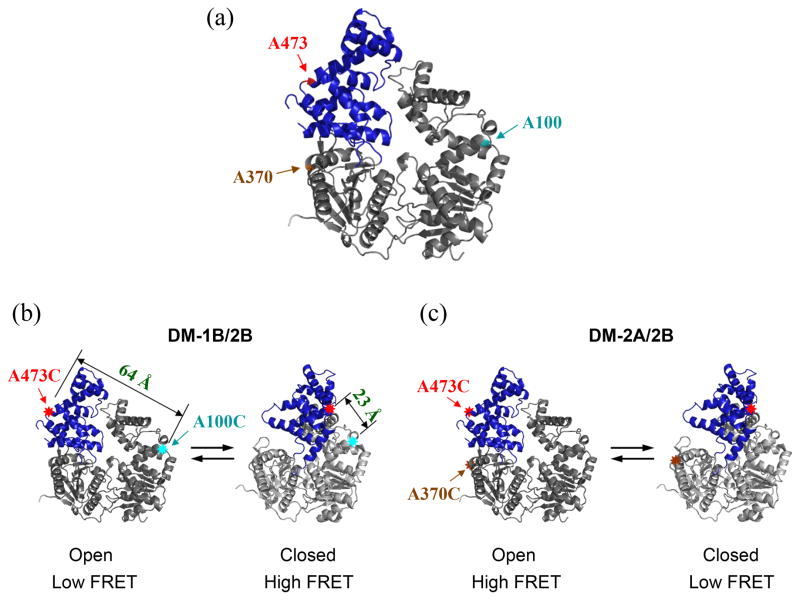
Figure 2. Design and fluorescent labeling of the UvrD mutants used to monitor the 2B sub-domain rotation by FRET.
Two different double Cys UvrD mutants, UvrDΔCys[A100C, A473C] (DM-1B/2B) and UvrDΔCys[A370C, A473C] (DM-2A/2B) were made by the substitution of alanine with cysteine at the indicated positions. (a) The positions of the three Ala to Cys mutations are indicated in the apo UvrD structure. (b) UvrD(DM-1B/2B) labeled with a mixture of Cy3 and Cy5 should show high FRET in the closed form and low FRET in the open form. The distances between A100C and A473C in the two conformations are also indicated. (c) UvrD(DM-2A/2B) labeled with a mixture of Cy3 and Cy5 should show low FRET in the closed form and high FRET in the open form.