Abstract
CD8+ T cells primed in the absence of CD4+ T cell help are programmed to produce TRAIL, which results in Death receptor (DR5) mediated apoptosis upon restimulation. Here, we studied whether these `helpless' effector CD8+ T cells are consigned to an apoptotic fate or whether their helpless program can be altered by inflammatory or growth cytokines. We found that helpless CD8+ T cells regained their full proliferative and functional capacity only when IL-2 was added to cell cultures, while IL-7 and IL-15, two common gamma chain cytokines associated with CD8+ T cell homeostasis and memory, could only partly restore secondary expansion in helpless CD8+ T cells. Recovery of functional CD8+ T cell immunity by IL-2 was concomitant with induction of IL2Rα (CD25) expression, downregulation of TRAIL, and the upregulation of anti-apoptotic molecules Bcl-2 and FLIP. The addition of IL-2 to helpless CD8+ T cells also interfered with DR5-mediated apoptosis induction, indicating that IL-2 affects several components of the TRAIL-DR5 pathway. Collectively, these data demonstrate that the helpless phenotype is not fixed, and that IL-2R signaling at the time of reactivation can play an important role in restoring CD8+ T cell function.
Keywords: T cells, cytotoxic, cytokines, CD8, TRAIL
1. Introduction
In the naïve host, antigen-specific CD8+ T cells are present in very low frequencies. Upon antigenic stimuli through infection or immunization, naïve CD8+ T cells undergo rapid clonal expansion that results in significant numbers of antigen-specific CD8+ T cells [1–4]. After having reached a plateau for several days, T cell responses contract, and more than 90% of the antigen-specific CD8+ T cells are eliminated via apoptosis. The surviving antigen-specific CD8+ T cells create a memory pool that provides durable protection when re-challenged [5,6]. A number of studies have demonstrated that a relatively short duration of antigenic stimulation commits naïve CD8+ T cells to clonal expansion and effector differentiation. These data indicate that initial antigen encounter launches a genetic program in CD8+ T cells that stably modifies the phenotype and function of their daughter cells (progeny) [7–11].
Signals provided by CD4+ T helper cells during CD8+ T cell priming are critical for the acquisition and maintenance of functional immunity. Although primary CD8+ T cell responses can proceed independently of CD4+ T cells, the lack of CD4+ T cells during CD8+ T cell priming results in loss of efficient CD8+ T cell recall responses [12–15]. One consequence of CD8+ T cell priming in the absence of T cell help is the acquisition of a transcriptional program that results in the upregulation of TNF related apoptosis inducing ligand (TRAIL) upon secondary antigen encounter. [16–18]. Those data showed that T cell receptor triggering is required for the expression of TRAIL in helpless CD8+ T cells. The expression of TRAIL in reactivated helpless CD8+ T cells results in Death Receptor 5 (DR5) mediated apoptosis, providing a mechanistic explanation for the poor CD8+ T cell immunity observed during recall responses. To date, it is not known if helpless CD8+ T cells are consigned to an apoptotic fate upon secondary challenge, or if signals delivered in trans can protect helpless CD8+ T cells from TRAIL/DR5 mediated death.
In this report we show that facets of the helpless program are not fixed and can be altered by γchain cytokines, in particular by IL-2. While IL-2, IL-7 and IL-15 provide survival signals sufficient to maintain effector function of restimulated helpless CD8+ T cells, only IL-2 had the capacity to restore robust secondary expansion. Consistent with an important role for the IL-2 signaling axis in regulating the helpless phenotype, we found that CD8+ T cells primed without CD4 help failed to upregulate CD25 in recall responses. Interestingly, the addition of IL-2, IL-7 and IL-15 to cultures during restimulation was sufficient to restore the expression of CD25. Moreover, common γchain signaling influenced the apoptotic program of helpless CD8+ T cells by regulating the expression of DR-5, Trail, Flip and Bcl-2. Lastly, we show that IL-2 signals protected both helpless and helped CD8+ T cells from DR-5 mediated apoptosis. Together, our data provide a molecular explanation how cytokines can influence the fitness of secondary CD8+ T cell responses.
2. Material and Methods
2.1. Mice and cell lines
C57BL/6J mice were purchased from the Jackson laboratory (Bar Harbor, ME). Mice were maintained at the La Jolla Institute for Allergy and Immunology under specific pathogen free conditions in accordance with guidelines by the Association for Assessment and Accreditation of Laboratory Animal Care International. Tap sufficient and deficient mouse embryo cell lines (MEC) expressing the human adenovirus type 5 early region 1 (Ad5E1) [19] were cultured in IMDM supplemented with 10% fetal calf serum, 50μM 2-mercaptoethanol, 2mM L-glutamine, 20U/mL penicillin and 20μg/mL streptomycin.
2.2. Immunization and antibody treatment
Mice were immunized subcutaneously in the flank with 1×107 irradiated (3000 rad) TAP−/−-Ad5E1-MECs. CD4+ cells were depleted on the first three days prior to immunization by intraperitoneal administration of 100 μg GK1.5 (CD4-depleted at the time of priming: `helpless'; untreated: `helped'). At day 3 post immunization, all mice were treated with GK1.5 [12].
2.3. Isolation and stimulation of CD8+ T cells
CD8+ T cells were purified from spleens and lymph nodes (LN) of TAP−/−-Ad5E1-MEC-immunized mice by negative selection with the MACS CD8 isolation kit (Miltenyi Biotec, Germany). Purity of isolated CD8+ T cells was confirmed by FACS analysis and was routinely greater than 95% with less than 0.1% CD4+ T cell contamination. CD8+ T cells were restimulated in vitro for 6 days with irradiated syngeneic TAP+/+-Ad5E1-MECs (10:1 ratio). Recombinant mIL-1β, hIL-2, mIL-4, mIL-5, mIL-6, mIL-7, mIL-10, mIL-12, mIL-15, mIL-18, mTNF-α, mIFN-γ, mIFN-α, mGM-CSF, DR5-Fc or Fas-Fc (Peprotech, New Jersey; PBL, New Jersey; R and D Systems, Minnesota) were added to cultures at the time of in vitro re-stimulation at indicated concentrations. In some experiments, CD8+ T cells from spleens and LN cells from TAP−/−-Ad5E1-MEC immunized mice were cultured with biotinylated anti-DR5 at 5μg/mL (clone MD5-1, eBioscience, California) for 30 minutes as previously described [20]. Purified streptavidin (Invitrogen, California) was subsequently added to cultures at 5μg/mL to cross link the anti-DR5 antibody. After 30 min of incubation, CD8+ T cells were cultured for 6 days with TAP+/+-Ad5E1-MECs (10:1 ratio) in the presence or absence of IL-2.
2.4. Enumeration of antigen-specific CD8+ T cells
Spleen and LN cells were incubated for 5 h with the E1B192–200 peptide or control peptide OVA257–264 (aa:VNIRNCCYI and aa:SIINFEKL, resp.; A&A labs LLC, California) at 5μg/mL final concentration in the presence of Brefeldin A (BDbiosciences, California) either directly ex vivo, or after in vitro culture. Cells were stained for surface expression of CD8 and CD44, fixed and permeabilized using Cytofix/Cytoperm kit (BDbiosciences, California) and stained for intracellular IFN-γ, or IFN-γ in combination with antibodies to CD25, CD122 or CD127 (eBioscience, California) according to manufacturer's protocol. The fold expansion of E1B192–200-specific CD8+ T cells was calculated by dividing the absolute number of IFN-γ+CD8+ T cells after in vitro culture by the absolute number of IFN-γ+CD8+ T cells placed into culture [12,16]. The cytolytic activity of E1B192–200-specific CD8+ T cells was determined by the JAM assay using 3H-thymidine labeled EL-4 as target cells as described before [12,21].
2.5. Real-time reverse transcription-PCR (RT-PCR)
Purified CD8+ T cells were stimulated with the E1B192–200 peptide for the indicated time, and total RNA was isolated using TriZol (Gibco BRL, Maryland) according to the manufacturer's protocol. RNA was reverse transcribed with the M-MLV reverse transcriptase (Gibco BRL, Maryland) using random hexamers (Gibco BRL, Maryland). Sequence-specific primers for murine IL-2Rα (CD25), TRAIL, TRAIL-R2/DR5, FLIP, BcL-2, IL-2, IL-7, IL-15, L32 and 18S were previously described [16]. Real Time PCR™ was performed with Amplitaq Gold™ polymerase in a PE biosystems 5700 thermocycler using SyBr Green™ detection protocol as outlined by the manufacturer. L32 and 18S were used as internal controls.
2.6. Statistical methods
Unless stated otherwise, data are expressed as mean ± standard error of the mean (S.E.M), and evaluated using a two-tailed analysis of variance (ANOVA) followed by a Dunnett test. A probability value of p<0.05 was considered statistically significant.
3. Results
3.1. Helpless effector CD8+ T cells die a TRAIL mediated death
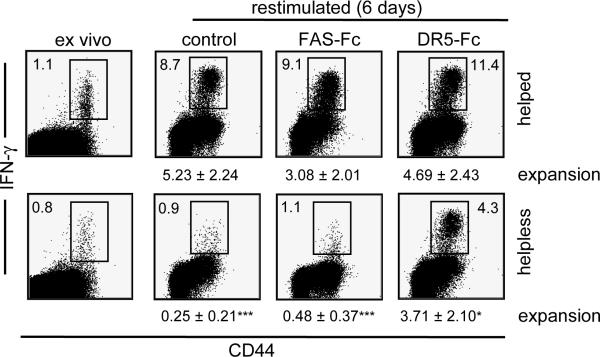
Immunization in the absence of CD4+ T cells results in compromised `helpless' CD8+ T cells that undergo TRAIL induced cell death upon secondary antigen encounter [12–16]. To study this in further detail, we immunized intact `helped' and CD4-depleted “helpless” mice with TAP-deficient MECs that were transfected with the human adenovirus type 5 early region 1 (designated TAP−/−-Ad5E1-MECs). Antigen-specific, IFNγ-producing CD8+ T cells were enumerated ex vivo one week after immunization, and secondary expansion of helped and helpless CD8+ T cells was assessed upon a six day in vitro restimulation with irradiated TAP+/+-Ad5E1-MECs [12,16,19]. Consistent with previous reports, helpless CD8+ T cells failed to expand upon secondary antigen encounter, as opposed to helped CD8+ T cells (Fig. 1). Importantly, the secondary expansion of helpless CD8+ T cells could be restored by blocking the TRAIL/DR5 pathway, but not by inhibiting the FAS/FASL interaction (p<0.05).
Figure 1. TRAIL regulates the secondary expansion of helpless CD8+ T cells.
Untreated (helped) or CD4 depleted (helpless) C57BL/6J mice were immunized with 1×107 irradiated TAP−/− Ad5E1 MECs. At day 7 after immunization CD8+ T cells were purified and cultured for 6 days together with TAP+/+-Ad5E1 MECs cells in the absence or presence of Fas-Fc or DR5-FC (5ug/ml). The frequency of antigen-specific CD8+ T cells ex vivo, and after in vitro restimulation was determined by quantifying E1B192-200-specific IFN-γ producing cells by flow cytometry. Data of one representative mouse of three mice is shown. The frequency of IFN-γ producing cells within the CD8+ T cell population is depicted within the FACS plot. Numbers below the FACS plots show secondary expansion of E1B192-200-specific IFN-γ producing CD8+ T cells at the end of the culture as mean ± S.E.M. of 3–4 mice per group (representative of 4 experiments). *** p<0.005 compared to helped CD8+ T cells; * P<0.05 compared to helpless CD8+ T cells).
3.2. Common γchain cytokines can restore secondary responses of helpless CD8+ T cells
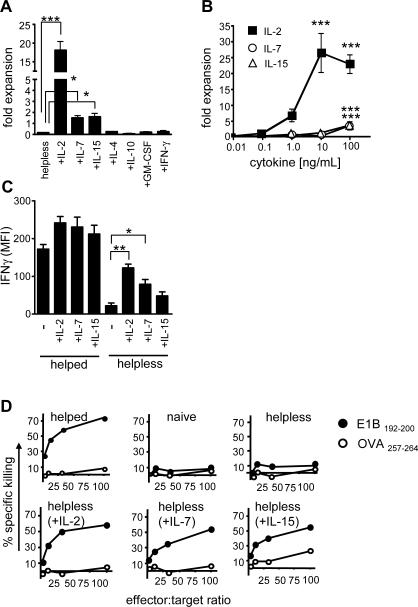
We next assessed if cytokines had the potential to alter the helpless T cell phenotype during secondary antigenic stimulation. To this end, we added a broad range of cytokines produced by lymphocytes or antigen presenting cells (APCs) to helpless CD8+ T cell cultures. The inflammatory cytokines IL-1β, TNF-α, IFNα and IFNβ, and the growth factors IL-6 and GM-CSF had no effect on viability and secondary expansion of helpless CD8+ T cells, whereas APC-derived proinflammatory IL-12 and IL-18 modestly improved survival (Fig. 2a, data not shown). The Th1-associated cytokine IFN-γ, and the Th2 associated cytokines IL-4, IL-5 and IL-10 provided little rescue. In contrast, the common γ chain cytokines IL-2, IL-7, and IL-15 could significantly improve survival and expansion of helpless CD8+ T cell cultures. Specifically, the addition of exogenous IL-2 (20ng/mL) markedly increased the secondary expansion of helpless CD8+ T cells (p<0.002), ultimately exceeding the expansion of helped CD8+ T cells (without exogenous IL-2) by 10-fold (Fig. 2a; data not shown). The addition of IL-7 (100ng/mL, p<0.02) and IL-15 (100ng/mL, p<0.05) also restored secondary expansion of antigen-specific helpless CD8+ T cells, albeit to a much lesser extent (Fig. 2a). The rescue of helpless CD8+ T cells was not a universal feature of all γc cytokines; IL-4 failed to protect helpless CD8+ T cells, at a dose range from 2–500ng/mL (Fig. 2a; data not shown).
Figure 2. Common γc cytokines IL-2, IL-7 and IL-15 can rescue the helpless phenotype.
Purified helped and helpless CD8+ T cells from mice immunized with TAP−/−-Ad5E1 MECs were isolated on day 7 post immunization and cultured with TAP+/+ -Ad5E1 MECs in the presence of the indicated cytokine. Unless indicated otherwise, cytokines were used at concentrations of 10ng/ml (IL-2, GM-CSF), or 200 ng/ml (IL-4,-7,-15,-10, IFN-γ). (A, B) After 6 days of in vitro culture the secondary expansion of E1B192-200-specific IFN-γ producing CD8+ T cells was assessed as described above (Fig. 1). (C) The mean fluorescence intensity (MFI) of IFNγ of in vitro restimulated E1B192-200-specific CD8+ T cells was determined after 6 days in vitro culture in the presence or absence of indicated cytokines. (D) Cytolytic activity of purified naive, or helped and helpless CD8+ T cells from immunized mice 6 days after restimulation with TAP+/+-Ad5E1 MECs in the presence of indicated cytokine. Cytolytic activity was assessed using the JAM assay by determining total loss in 3H-thymidine from E1B192-200-pulsed or OVA257-264-pulsed (control) target cells. Data are presented as mean ± S.E.M. * p<0.05; ** p<0.02; *** p<0.005; (n= 3–4 mice per group; repeated 3 times).
Examination of a dose response curve to IL-2, IL-7 and IL-15 revealed that as little as 1ng/mL of IL-2 added at the time of antigenic restimulation resulted in a 5-fold expansion of helpless CD8+ T cells over control cells (i.e. helped CD8+ T cells cultured without IL-2), with a maximal expansion between 10–100ng/mL (Fig. 2b, p<0.0001). For IL-7 and IL-15, 100ng/ml was required to achieve a more modest secondary expansion that was below the expansive capacity of helped CD8+ T cells (data not shown).
IL-2, IL-7 and IL-15 were also capable to maintain the effector function of restimulated helpless CD8+ T cells. We found that IL-2 and IL-7 significantly restored the IFNγ production of helpless CD8+ T cells on a per cell basis (Fig. 2c). Furthermore, helpless CD8+ T cells regained their capacity to kill target cells after 6 days of in vitro restimulation (Fig. 2d). The marked increase of cytolytic function resembled that of helped CD8+ T cells, in particular when IL-2 was added to the culture. In contrast, helpless CD8+ T cells that were restimulated without cytokines exhibited cytotoxicity levels that were similar to that of naïve polyclonal CD8+ T cells (Fig. 2d) [12]. These data indicate that IL-2, IL-7 and IL-15 have the capacity to maintain the effector function of helpless CD8+ T cells, however, IL-2 alone is sufficient to support full secondary expansion. Together, this suggests that IL-2, IL-7 and IL-15 play overlapping and divergent roles in the restoration of secondary expansion and maintenance of effector function of helpless CD8+ T cells in vitro.
3.3. Regulation of CD25 expression by common γchain cytokines
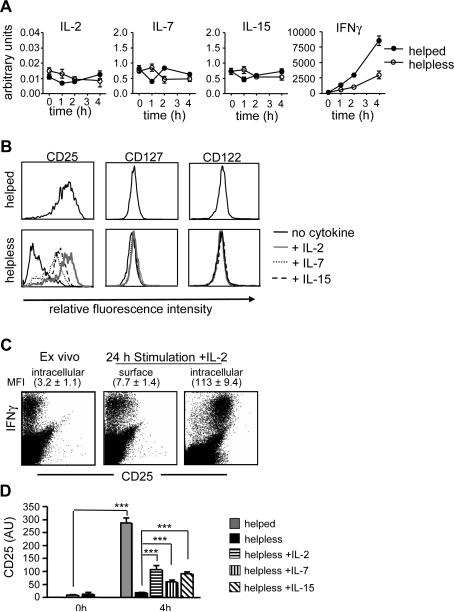
We next sought to determine how IL-2, IL-7, and IL-15 restore helpless CD8+ T cell expansion during secondary antigen encounter. Gene expression studies indicated that both helped and helpless CD8+ T cells express similar levels of IL-2, IL-7, and IL-15 in response to antigenic stimulation (Fig. 3a). Hence, it appears unlikely that restoration of secondary expansion and effector function by cytokines is the product of an autocrine feedback loop. Therefore, we considered the possibility that common γchain receptor signaling was altered in helpless CD8+ T cells. To address this hypothesis, we first examined the expression pattern of the common γc cytokine receptors on helped and helpless CD8+ T cells ex vivo. After 24 and 48 hours of in vitro restimulation, we could not detect differences in the expression levels of the IL-2/15 shared β chain (CD122) and the IL-7Rα chain (CD127) on antigen-specific CD8+ T cells (Fig. 3b and data not shown). In contrast, helpless CD8+ T cells expressed substantially lower levels of the high affinity IL-2Rα chain (CD25) after antigenic stimulation (MFI 441 v 21, Fig. 3b). The addition of IL-2 during antigenic restimulation restored CD25 expression on helpless CD8+ T cells that was comparable to reactivated helped CD8+ T cells. We also observed modest increase in CD25 levels on helpless CD8+ T cells upon addition of IL-15, and to a lesser extent of IL-7 (MFI 148 and 106, respectively).
Figure 3. Modulation of IL-2 receptor by IL-2 on helpless CTL.
(A) mRNA expression of IL-2, IL-7, IL-15 and IFNγ in purified helped or helpless CD8+ T cells that were restimulated with E1B192-200 peptide for indicated time points. (B) Purified helped or helpless CD8+ T cells were cultured with TAP+/+-Ad5E1 MECs for 24h in the presence of indicated cytokines (IL-2; 20ng/ml, IL-7 and IL-15; 200ng/ml). Total expression of IL-2Rα (CD25), IL-7R (CD127), and IL-15R subunit IL-2Rβ (CD122) of IFNγ-producing CD8+ T cells after an additional 7 h restimulation with E1B192-200 peptide in the presence of Brefeldin A. (C) Cell surface and total (surface and intracellular) CD25 expression in E1B192-200-specific IFNγ producing helpless CD8+ T cells 24h after stimulation. Staining was performed after an additional 7h restimulation with E1B192-200 peptide in the presence of Brefeldin A. Numbers represent the mean ± S.E.M. in fluorescent intensity for the CD25 stain (3 mice per group). (D) Expression of CD25 mRNA upon 4 h culture with E1B192-200 peptide as determined by quantitative RT-PCR. Relative expression values are presented normalized to L32. Data in A and B are representative of 2 experiments, both composed of 3 mice/group. Data in C and D are presented as mean ± S.E.M. *** p<0.005 (n=3–4 mice per group, representative of 3 experiments).
The expression of CD25 on helped CD8+ T cells upon antigenic re-stimulation appears to be regulated by de novo synthesis of CD25 rather than by redistribution of preformed protein. This is reflected by the fact that restimulation of CD8+ T cells in the presence of Brefeldin A resulted in significant accumulation of CD25 protein in the endoplasmic reticulum and that surface CD25 expression remained unaltered (Fig 3b,c). In addition, quiescent helpless CD8+ T cells had little or no detectable surface and intracellular CD25 expression directly ex vivo (Fig. 3c, left panel). This also correlated with low CD25 mRNA levels (Fig. 3d). Activation of helpless CD8+ T cells, however, led to rapid production of CD25 transcripts (p<0.001). The induction of CD25 transcripts was specific for helped CD8+ T cells, as helpless CD8+ T cells failed to upregulate CD25 message and protein upon antigenic restimulation (Fig. 3b, d). This transcriptional defect could be modestly restored by adding IL-2, IL-7, and IL-15 to the culture (Fig. 3d; p<0.005) and correlated with increased expression of CD25 protein in the cells (Fig. 3b). Thus, the differential regulation of CD25 as a consequence of the presence or absence of CD4+ T cell help at priming suggests that CD25 expression, like TRAIL expression, is part of the transcriptional program of helpless CD8+ T cells.
3.4. Modifications of the helpless program by IL-2, IL-7, and IL-15
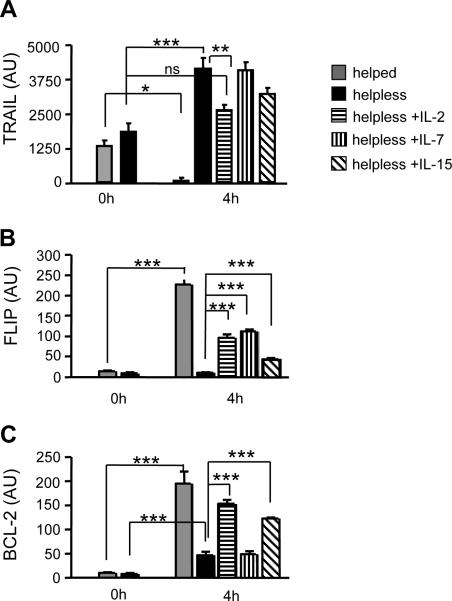
The key characteristic feature of the helpless transcriptional program is TRAIL-mediated activation-induced cell death (AICD) that is reflected by induction of TRAIL mRNA upon restimulation exclusively in helpless, but not helped CD8+ T cells ([16]; Fig 4a, p<0.0001). Given that IL-2, IL-7 and IL-15 can restore helpless CD8+ T cell responses, we addressed whether they can also modify TRAIL expression. As we have recently shown, IL-2 (20ng/mL) significantly decreased the induction of TRAIL mRNA in helpless CD8+ T cells (Wolkers et al., manuscript in preparation and Fig. 4a, p<0.02). The addition of IL-15 (100ng/mL) also resulted in a modest, but not significant downregulation of TRAIL in helpless CD8+ T cells, while IL-7 did not affect TRAIL induction (at a concentration sufficient to partly restore secondary expansion and effector function; 100ng/mL, Fig. 2).
Figure 4. IL-2 regulates the helpless program.
Purified CD8+ T cells from helped, or helpless immunized mice were stimulated with E1B192-200 peptide and indicated cytokines (IL-2; 20ng/ml, IL-7 and IL-15; 200ng/ml). mRNA was extracted 0 and 4 h after peptide stimulation. Expression of TRAIL (A), FLIP (B), and Bcl-2 (C) were determined by quantitative RT-PCR. Data are presented as mean ±S.E.M. *P<0.05; ** p<0.02, *** p<0.005 (n= 3–4 mice per group; representative of 3 experiments).
Because IL-2 significantly, but not completely, suppressed TRAIL expression in reactivated helpless CD8+ T cells, we hypothesized that IL-2R signaling may prevent AICD by additional effects, i.e. by inducing anti-apoptotic genes. The Flice inhibitory protein (FLIP) can inhibit Death Receptor mediated AICD by blocking the intracellular caspase cascade [22]. Flip is induced in helped CD8+ T cells after antigenic stimulation, but not in helpless CD8+ T cells (Fig. 4b, p<0.001) [16]. Strikingly, all three common γ chain cytokines restored the induction of FLIP mRNA upon reactivation in helpless CD8+ T cells (Fig. 4b, p<0.001). Similarly, the anti-apoptotic factor Bcl-2 that can decrease the susceptibility to TRAIL mediated AICD [23] was upregulated in restimulated helpless CD8+ T cells upon the addition of IL-2 and IL-15 (p<0.001), but not by IL-7 (Fig. 4c). Together, our findings indicate that IL-2, IL-7, and IL-15 employ several pathways to decrease susceptibility to AICD in helpless CD8+ T cells. These include the reduction of TRAIL expression, and the concomitant induction of Bcl-2 and FLIP, the latter possibly interfering with the DR5 signaling pathway and/or decreasing DR5 sensitivity.
3.5. IL-2 protects helpless CD8+ T cells from DR5 mediated apoptosis
The upregulation of anti-apoptotic genes in response to exogenous cytokines led us to hypothesize that IL-2R signaling may directly influence TRAIL-DR5 mediated cell death. Indeed, administration of IL-2 (at 20ng/mL) during antigenic restimulation resulted in a significant decrease in DR5 mRNA levels in helpless CD8+ T cells (p=0.002), while DR5 mRNA in helped CD8+ T cells appeared unchanged (Fig. 5a). This divergent expression pattern of DR5 in helped and helpless CD8+ T cells was only evident when IL-2 was added during restimulation, because DR5 mRNA expression was comparable directly ex vivo and upon peptide restimulation alone (Fig. 5a). Of note, DR5 and its downstream signaling is fully functional in helped CD8+ T cells, as restimulation in the presence of recombinant TRAIL completely abrogated secondary expansion (data not shown, and [16]).
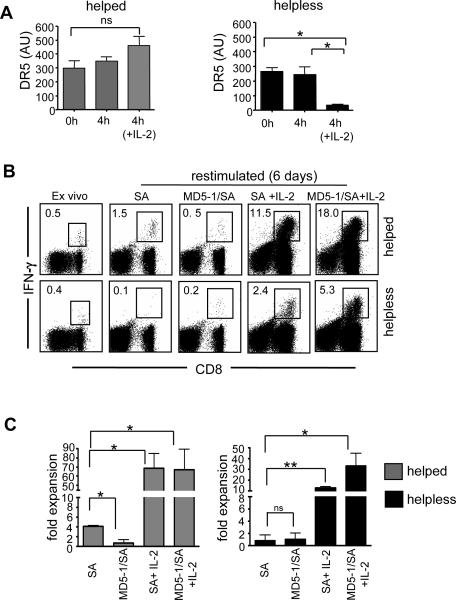
Figure 5. IL-2 regulates the susceptibility of CD8+ T cells to DR5 mediated death.
(A) Helped (left panel) and helpless (right panel) CD8+ T cells were purified from immunized mice and DR5 expression levels were determined by quantitative RT-PCR from after 4h stimulation with, or without E1B192-200 peptide stimulation in the absence or presence of IL-2 (20ng/ml). Relative expression values are presented normalized to L32. (B, C) Helped and helpless splenocytes isolated from immunized mice were pretreated for 1h with complete media and streptavidin alone (SA), or streptavidin and biotinylated anti-DR5 (clone MD5-1) antibody. Cells were then cultured with TAP+/+-Ad5E1 MECs for 6 days in the absence or presence of IL-2 (20ng/ml), and the frequency and fold expansion of E1B192-200 -specific CD8+ T cells was determined as described above. (B) Flow cytometry data from one representative experiment. (C) Compiled data from 3 biological replicates. All experiments are representative of 4 independent experiments composed of 3 mice/group. Data in A and C are presented as mean ± S.E.M. * p<0.05; ** p<0.02.
To directly test whether IL-2 protects helpless CD8+ T cells from DR5 mediated cell death, we pretreated helped and helpless CD8+ T cells with the cross-linked agonistic anti-DR5 antibody MD5-1 [20] before the CD8+ T cells were restimulated in vitro. As expected, helpless CD8+ T cells did not expand when treated with cross-linked MD5-1, confirming that MD5-1 is not acting as a DR5 blocking antibody in this system (Fig. 5b, c). Interestingly, helped CD8+ T cells cultures were significantly affected by cross-linked MD5-1 as assessed by their reduced frequency and total number of IFN-γ+CD8+ T cells (p<0.02), further confirming that helped CD8+ T cells maintain their susceptibility to TRAIL/DR5 mediated death. Importantly, the addition of 20ng/ml IL-2 to cultures drove secondary expansion in both helped and helpless CD8+ T cells regardless of MD5-1 treatment (Fig. 5b, c; p<0.05). These data indicate that IL-2 signals interfere with DR-5 mediated apoptosis both by decreasing the ligand production and by reducing the sensitivity to DR5-mediated cell death.
4. Discussion
CD8+ T cells primed in the absence of CD4 help fail to undergo robust secondary expansion when reactivated by cognate antigen. In this report, we sought to determine if helpless CD8+ T cells are consigned to TRAIL-mediated cell death, or if signals delivered in trans could alter the helpless program. In support of the latter assertion, we found that IL-2, IL-7 and IL-15 significantly influenced helpless CD8+ T cell fate and function. These data indicate that the genetic and functional program acquired by helpless CD8+ T cells during priming can be overwritten to some extent at the time of secondary antigen encounter if the correct signals are delivered.
It has previously been shown that the common γ chain receptor is critical for CD8+ memory T cell formation [24]. In particular, IL-2 signals during T cell priming are pivotal for proficient CD8+ T cell memory formation, both in terms of expansion and differentiation after secondary challenge [25]. Moreover, IL-15 and recently IL-21 have been shown to provide `help' signals during CD8+ T cell priming [18,26]. Here, we extend these observations to the secondary expansion phase of CD8+ T cells and demonstrate that the common γc cytokines IL-2, IL-7 and IL-15 preserve effector functions in reactivated helpless CD8+ T cells.
We observed that IL-2, and to a lesser extent IL-7 and IL-15, modulates the expression of several genes involved in apoptosis including Bcl-2, Flip, Trail and DR-5. Therefore, it appears likely that a combination rather than one single gene mediates the rescue of helpless CD8+ T cells to significantly reduce TRAIL mediated death and DR5 sensitivity. Consistent with this idea, Bcl-2 transgenic overexpression by itself (that is induced in reactivated helpless CD8+ T cells by IL-2 and IL-15 (Fig. 4c)) could not restore secondary expansion in helpless CD8+ T cells [16].
Interestingly, while all three cytokines could equally well restore effector functions such as IFNγ production and cytotolytic activity, only IL-2 had the capacity to profoundly restore the secondary expansion in helpless CD8+ T cells. This differential outcome was also reflected in their distinct effects on TRAIL production, sensitivity to DR5 signaling and CD25 expression. These data suggest that cytokines involved in lymphocyte maintenance (e.g. IL-7 and IL-15) may play an important role in allowing helpless cells to persist, but IL-2 appears to be the dominant cytokine in reprogramming helpless CD8+ T cells to fully functional effector cells during antigenic restimulation. Therefore, it will be of interest to determine if helpless CD8+ T cells that have been reactivated in the presence of IL-2 maintain the helpless transcriptional program for their progeny, or if IL-2 can rewrite the CD8 program to ensure long-term CD8+ T cell immunity.
The molecular signature of CD8+ T cell immunity is not fully determined yet. Here we show that the high affinity IL-2 receptor α unit (CD25) is an important component of secondary expansion. While the CD25 expression is low in helped and helpless CD8 T cells directly ex vivo, CD25 levels were significantly increased by cytokines in reactivated helpless CD8+ T cells, in particular by IL-2 via its IL-2/IL-2R positive feedback loop. Recent data have provided evidence that CD4+ T cell help during T cell priming increases CD25 expression on CD8+ T cells indirectly through CD40L engagement on APCs [27,28]. We now show that high CD25 expression levels on CD8+ T cells also appear pivotal during reactivation to provide proficient secondary CD8+ T cell responses. Furthermore, our study indicates that regulation of CD25 expression is part of the transcriptional program of helpless CD8+ T cells. Therefore, we identified CD25 as a second marker to distinguish helped from helpless CD8+ T cells ex vivo, in addition to TRAIL expression and AICD upon restimulation of helpless CD8 T cells.
We found that antigenic restimulation of helpless CD8+ T cells in vivo did not result in increased expression of CD25 (EM Janssen; unpublished data), suggesting that circulating IL-2 levels are not sufficient to induce CD25 expression on the CD8+ T cells. In light of these findings, it is tempting to speculate that administration of IL-2 in vivo may restore helpless CD8+ T cell responses, and that current IL-2 based therapies mediate their effects through the modification of a relatively “helpless” program [29–31]. In conclusion, the data presented herein provide important insights into the fate and function of CD8+ T cells primed in the absence of CD4 T cell help and could have important therapeutic implications for vaccine strategies to infectious disease and cancer.
Research highlights
CD8 T cells require CD4 T cell help for proficient memory formation.
Addition of cytokines rescues `helpless' CD8 T cells from TRAIL mediated cell death.
Cytokines change the transcriptional profile of `helpless' CD8 T cells.
Acknowledgements
This work was supported in part by a Career Development Award from the Leukemia and Lymphoma Society (#3248-05 to E.J.), the National Institutes of Health (#RR021975 to S.B.), and the Cancer Research Institute/Irvington Institute (M.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Callan MF, Tan L, Annels N, Ogg GS, Wilson JD, O'Callaghan CA, Steven N, McMichael AJ, Rickinson AB. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus In vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Doherty PC. The numbers game for virus-specific CD8+ T cells. Science. 1998;280:227. doi: 10.1126/science.280.5361.227. [DOI] [PubMed] [Google Scholar]
- [4].Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- [5].Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- [6].Sprent J, Tough DF. T cell death and memory. Science. 2001;293:245–248. doi: 10.1126/science.1062416. [DOI] [PubMed] [Google Scholar]
- [7].van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- [8].van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- [9].Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8+ T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- [10].Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Teixeiro E, Daniels MA, Hamilton SE, Schrum AG, Bragado R, Jameson SC, Palmer E. Different T cell receptor signals determine CD8+ memory versus effector development. Science. 2009;323:502–505. doi: 10.1126/science.1163612. [DOI] [PubMed] [Google Scholar]
- [12].Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- [13].Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- [14].Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- [15].Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- [17].Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- [18].Oh S, Perera LP, Terabe M, Ni L, Waldmann TA, Berzofsky JA. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc Natl Acad Sci U S A. 2008;105:5201–5206. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schoenberger SP, van der Voort EI, Krietemeijer GM, Offringa R, Melief CJ, Toes RE. Cross-priming of CTL responses in vivo does not require antigenic peptides in the endoplasmic reticulum of immunizing cells. J Immunol. 1998;161:3808–3812. [PubMed] [Google Scholar]
- [20].Takeda K, Yamaguchi N, Akiba H, Kojima Y, Hayakawa Y, Tanner JE, Sayers TJ, Seki N, Okumura K, Yagita H, Smyth MJ. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J Exp Med. 2004;199:437–448. doi: 10.1084/jem.20031457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Methods. 1991;145:185–192. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- [22].Budd RC, Yeh WC, Tschopp J. cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol. 2006;6:196–204. doi: 10.1038/nri1787. [DOI] [PubMed] [Google Scholar]
- [23].Guo BC, Xu YH. Bcl-2 over-expression and activation of protein kinase C suppress the trail-induced apoptosis in Jurkat T cells. Cell Res. 2001;11:101–106. doi: 10.1038/sj.cr.7290074. [DOI] [PubMed] [Google Scholar]
- [24].Decaluwe H, Taillardet M, Corcuff E, Munitic I, Law HK, Rocha B, Riviere Y, Di Santo JP. Gamma(c) deficiency precludes CD8+ T cell memory despite formation of potent T cell effectors. Proc Natl Acad Sci U S A. 2010;107:9311–9316. doi: 10.1073/pnas.0913729107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Barker BR, Gladstone MN, Gillard GO, Panas MW, Letvin NL. Critical role for IL-21 in both primary and memory anti-viral CD8+ T-cell responses. Eur J Immunol. 2010;40:3085–3096. doi: 10.1002/eji.200939939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci U S A. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wiesel M, Joller N, Ehlert AK, Crouse J, Sporri R, Bachmann MF, Oxenius A. Th cells act via two synergistic pathways to promote antiviral CD8+ T cell responses. J Immunol. 2010;185:5188–5197. doi: 10.4049/jimmunol.1001990. [DOI] [PubMed] [Google Scholar]
- [29].Overwijk WW, Theoret MR, Restifo NP. The future of interleukin-2: enhancing therapeutic anticancer vaccines. Cancer J Sci Am. 2000;6(Suppl 1):S76–80. [PMC free article] [PubMed] [Google Scholar]
- [30].McDermott DF, Atkins MB. Immunotherapy of metastatic renal cell carcinoma. Cancer J. 2008;14:320–324. doi: 10.1097/PPO.0b013e31818675c4. [DOI] [PubMed] [Google Scholar]
- [31].Boyman O, Surh CD, Sprent J. Potential use of IL-2/anti-IL-2 antibody immune complexes for the treatment of cancer and autoimmune disease. Expert Opin Biol Ther. 2006;6:1323–1331. doi: 10.1517/14712598.6.12.1323. [DOI] [PubMed] [Google Scholar]