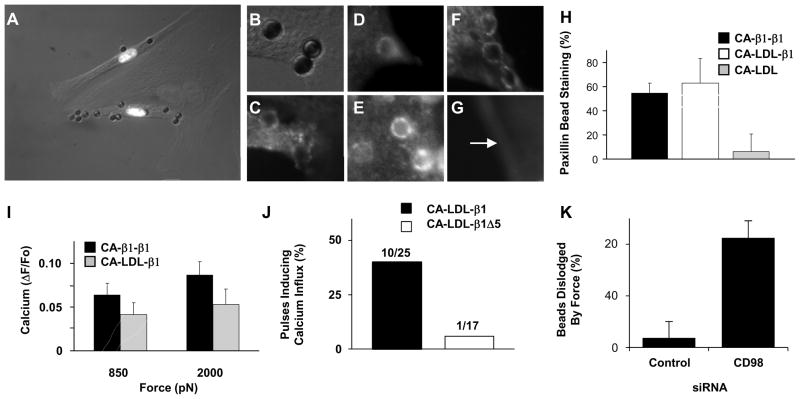
Fig. 3. Distal integrin cytoplasmic domain is necessary for rapid force-induced calcium signaling.
(A) Merged phase contrast and immunofluorescence photomicrographs of three BCE cells demonstrating that only cells transfected with the CA-β1-β1 construct bind beads coated with anti-CA antibody (white nuclei indicate cells transfected with chimera and histone 2B Ds-red; an adjacent non-transfected cells is outlined in white). (A, C) High magnification phase contrast (B) and fluorescence (C) view of beads shown in A after cells expressing CA-β1-β1 were stained for paxillin (C). Fluorescence imaging of GFP-talin (D) and actin (E), in similarly treated cells. (F, G) Fluorescence views of beads in cells transfected with CA-LDL-β1 (F) or CA-LDL (G) stained for paxillin. Arrow indicates bead position. (H) Quantitation of paxillin staining results, and of calcium signaling responses (I), for the different integrin chimeras. (J) Percentage of 3 sec tensional force pulses (350 pN) applied via anti-CA antibody coated microbeads to BCE cells transfected with the indicated integrin chimeras inducing calcium influx. (K) Percentage of RGD coated microbeads bound to BCE cells treated with indicated siRNA that were dislodged by 2000 pN force.