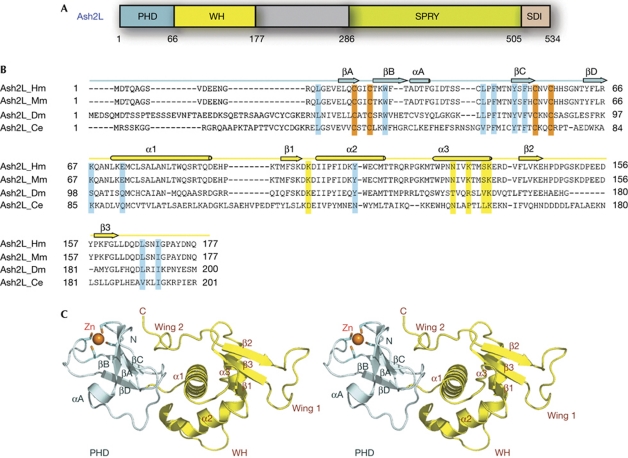
Figure 1.
Crystal structure of the N-terminal domain of Ash2L. (A) Domain organization of Ash2L. The PHD finger is coloured in pale cyan, the winged helix (WH) motif in yellow, the SPRY domain in green and the SDI (Sdc1/DPY30 interaction) motif in pink. (B) Sequence alignment of the N-terminal regions of four Ash2L family members. Secondary structure assignments based on the human Ash2L crystal structure are shown as coloured cylinders (α-helices) and arrows (β-strands) above the sequences. The cysteine residues involved in zinc binding are shown in orange. The putative residues involved in DNA binding are highlighted in yellow, and the residues important for the interaction between the PHD and WH motifs are highlighted in cyan. (C) Stereoview of Ash2LNTD. The PHD finger is coloured in pale cyan and the WH domain in yellow. The zinc ion in sphere representation and the zinc-binding cysteine residues are shown in stick models. Zn, zinc.