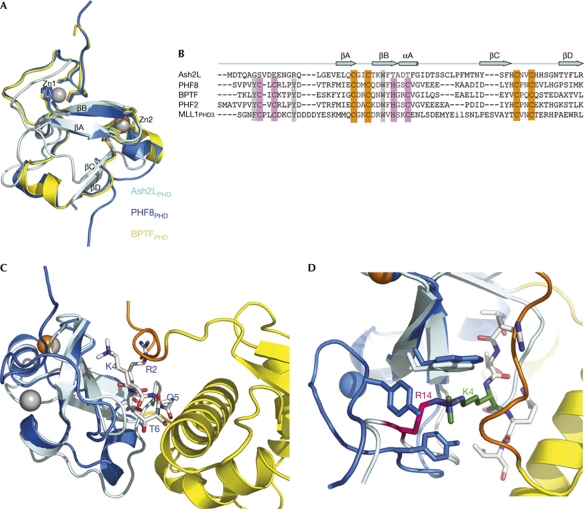
Figure 2.
Ash2LPHD is an atypical PHD finger. (A) Superimposition of Ash2LPHD (cyan), PHF8PHD (marine) and BPTFPHD (yellow). There are two zinc ions in the PHF8PHD structure (grey), but only one zinc ion in Ash2LPHD (orange). (B) Structure-based sequence alignment of Ash2LPHD with four PHD fingers that share high structural similarity. The conserved zinc-binding residues are highlighted in orange, the absent zinc-binding residues in pink and the absent aromatic cage residues in grey. (C) Structural superimposition of Ash2LPHD (cyan) and the PHF8PHD–H3 peptide complex (marine). H3 peptide is shown in sticks (grey). The carboxy-terminal wing 2 from Ash2LWH is shown in orange. (D) The R14 of Ash2L (magenta) and the K4 of H3 (green) occupy the same pocket surrounded by aromatic residues. WH, winged helix; Zn, zinc.