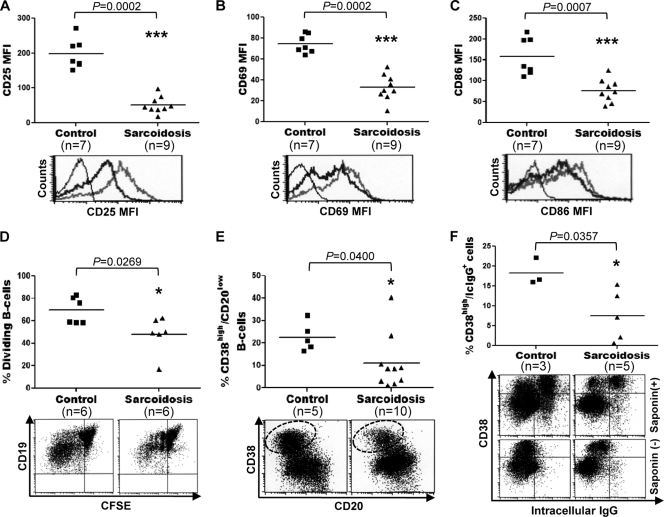
Fig. 3.
Impaired activation, proliferation, and differentiation of sarcoid B cells. (A to C) CD19+ lymphocytes, purified from PBMC of sarcoid patients and healthy controls by magnetic-activated cell sorting, were incubated for 24 h with CD40L, CpG-ODN, IL-2, IL-10, and IL-15. (Top) The cells were then analyzed by FC for levels of expression of CD25 (A), CD69 (B), and CD86 (C). Horizontal lines indicate median MFI values. (Bottom) Representative histograms. Thin lines, isotype control; thick black lines, sarcoidosis cells; thick gray lines, control cells. (D and E) Blood CD19+ cells, purified either from sarcoidosis patients or from healthy controls, were labeled with CFSE and were stimulated with CD40L, CpG-ODN, IL-2, IL-10, and IL-15 for 5 days. The frequency of dividing B cells (D) and plasma cells (CD20low CD38high) (E) were determined by FC in the CD19+ population. Representative dot plots are shown below the graphs. Plasma cells are shown within dotted ovals in the dot plot in panel E. (F) Purified preparations of CD19+ cells from sarcoid patients and healthy controls were first stimulated for 4 days as described for panels D and E and then restimulated with IL-2, IL-10, and IL-15 for 3 days. At day 7, the frequency of IgG-containing cells in these cultures was determined by intracellular staining using FC. Upon saponin permeabilization, CD20low CD38high cells were specifically labeled for intracellular (Ic) IgG (top). No intracellular IgG staining was observed in CD20low CD38high cells without saponin permeabilization (bottom).