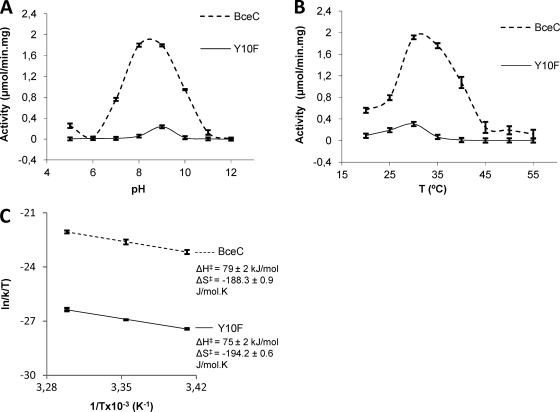
Fig. 1.
Dehydrogenase activities of BceC and its Y10F mutant. (A) Activity relative to the pH of the medium at 30°C. (B) Activity relative to the temperature of the medium at pH 8.7. (C) Plot of the first three values of ln(k/T) versus 1/T (the ignored remaining values correspond to degraded conformations of the enzyme at higher temperatures) allows an estimation of ΔH‡ (enthalpy of activation) and ΔS‡ (entropy of activation) according to the Eyring-Polanyi equation: ln(k/T) = −ΔH‡/R × 1/T + ln(kB/h) + ΔS‡/R, where kB is the Boltzmann constant, h is Planck's constant, and R is the gas constant (18, 20). Bars represent maximal intervals of (two or three) the measurements replicates.