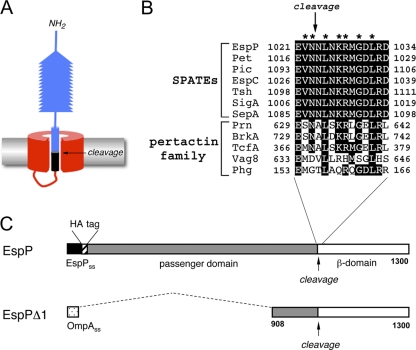
Fig. 1.
The sequence surrounding the passenger domain cleavage site is conserved in SPATEs and pertactin-like proteins. (A) Illustration of the structure of SPATE and pertactin-like proteins prior to passenger domain cleavage (based on references 3 and 35). The passenger domain is separated from the β domain by an intrabarrel proteolytic reaction following its translocation across the OM. (B) Sequence of the α-helical segment surrounding the passenger domain cleavage site in SPATE and pertactin-like proteins. Highly conserved residues are shaded black. Residues that were mutated in EspP in this study (and that were also mutated in Tsh in reference 19) are denoted with an asterisk. (C) Schematic representation of EspP and EspPΔ1. EspP contains a 55-residue signal sequence (SS) and a 968-residue passenger domain. The version of EspP that we studied here also contains an N-terminal HA tag. EspPΔ1 contains the OmpA signal sequence (which is functionally indistinguishable from the native EspP signal peptide [see reference 34]) and only the last 116 residues of the EspP passenger domain.