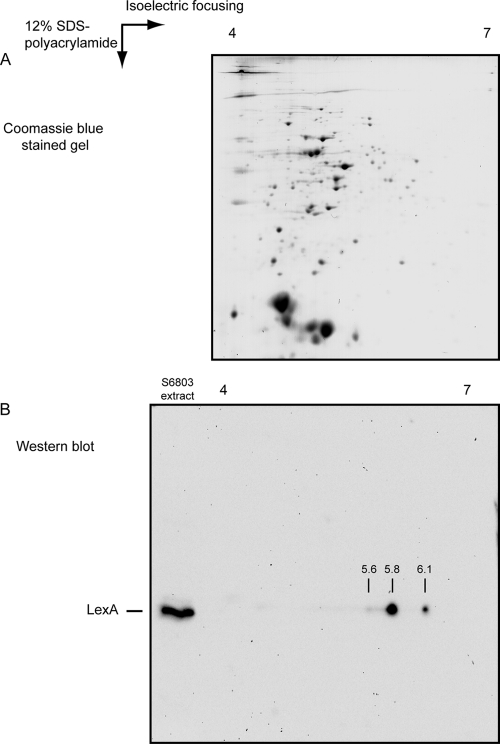
Fig. 3.
LexA in Synechocystis sp. strain PCC 6803 possesses different isoelectric points. (A) Coomassie blue-stained 2D gel with approximately 40 μg total protein from Synechocystis sp. strain PCC 6803 grown photoautotrophically in BG11 supplemented with 10 mM HEPES, pH 7.5, and bubbled with air at 25°C. As the first step, proteins were separated according to their pIs, using a strip with a pH gradient between 4 and 7, which was followed by the second separation on a 12% SDS-polyacrylamide gel. (B) Western blot analysis of the multiple LexA forms. A 2D separation of proteins was performed as shown in panel A prior to the Western blotting. On the same SDS-polyacrylamide gel, 5 μg total protein extract was loaded and separated on the left side of the gel as a control of the position of LexA. Using this approach, at least three different forms of LexA could be detected, with pIs of approximately 5.6, 5.8, and 6.1.