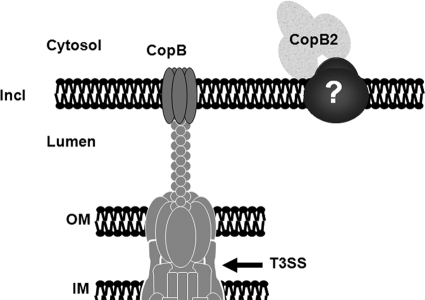
Fig. 9.
A working model for association of CopB and CopB2 with inclusion membranes. As T3S substrates, both CopB and CopB2 are secreted across the chlamydial inner and outer membranes (IM and OM, respectively) via the T3S apparatus to gain access to the inclusion membrane (Incl). CopB is predicted to form a prototypical translocation pore based on homology to YopB class translocator proteins and detection of endogenous CopB colocalization with the inclusion membrane (14). Association of CopB2 with the cytosolic face of inclusion membranes, however, is likely peripheral. Both the C-terminal and internal coiled-coil domains are involved in interactions with a chlamydial protein that is likely integral to the inclusion membrane.