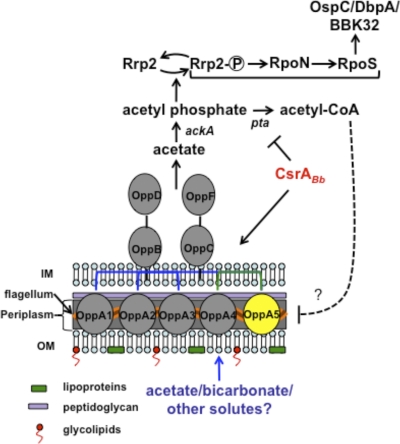
Fig. 9.
A model for the role of BBA34 in modulating the vertebrate host-specific adaptation in B. burgdorferi. We propose that BBA34/OppA5 facilitates the transport of acetate, which is converted to acetyl phosphate by acetate kinase. The increased accumulation of acetyl phosphate in turn facilitates the phosphorylation of Rrp2, leading to the activation of the Rrp2-RpoN-RpoS pathway under fed-tick or mammalian host-specific conditions (41, 70, 82, 88). Since CsrABb is upregulated under fed-tick conditions, leading to repression of phosphate acetyl transferase (Pta), there is increased accumulation of acetyl phosphate, resulting in sustained activation of the Rrp2-RpoN-RpoS pathway. Under conditions where the levels of CsrABb are reduced, there is a lack of repression of Pta, leading to the increased accumulation of acetyl-CoA, which in turn leads to reduced levels of expression of BBA34/OppA5. This latter proposition is based on our previous finding that the csrABb deletion mutant had reduced levels of BBA34, suggesting that acetyl-CoA could play a role as a key metabolite in modulating the pathways for the adaptation of spirochetes to vertebrate host-specific conditions. The model is skewed toward assuming acetyl phosphate as a major substrate contributing to the activation of the Rrp2-RpoN-RpoS pathway.