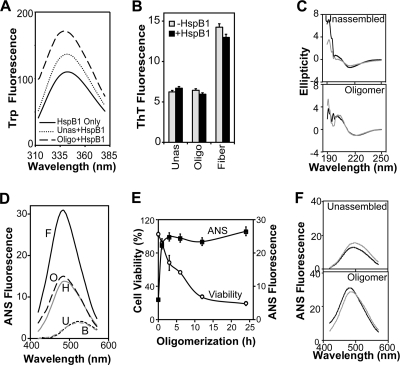
Fig. 3.
Characterization of the effect of HspB1 on Aβ conformers. (A) Interaction between Aβ conformers as indicated (10 μM as monomers) and HspB1 (2 μM as monomers) was monitored by the fluorescence properties of the tryptophans in HspB1. Fluorescence spectra were recorded between 310 and 370 nm after excitation at 295 nm in the absence of Aβ (HspB1 Only) or in the presence of unassembled Aβ (Unas) and oligomers (Oligo). (B) Effect of HspB1 on Aβ conformers was monitored by the amyloid-specific fluorescent dye thioflavin T (ThT; 20 μM). Excitation was at 420 nm, and emission was monitored at 450 to 550 nm. Fluorescence values at 484 nm of samples before (−HspB1) or after (+HspB1) coincubating Aβ conformers (10 μM as monomers) with HspB1 (2 μM as monomers) are plotted. (C) Secondary structural change in Aβ before (gray lines) or after (black lines) coincubation with HspB1 was monitored by circular dichroism spectroscopy between 190 and 250 nm. Ellipticity values shown are mean residue weight molar ellipticity (×1,000) with the unit degree·cm2·dmol−1. (D) Fluorescence studies using an extrinsic fluorophore, ANS, to detect conformational change in Aβ conformers. Aβ peptide was at 10 μM in all samples, and HspB1 was at 2 μM. ANS (10 μM) fluorescence was recorded between 420 and 570 nm after excitation at 370 nm. B, buffer; U, unassembled; O, oligomer; H, HspB1; F, fiber. (E) Kinetics of Aβ oligomer formation was monitored by ANS fluorescence change (y axis on right) and acquisition of cytotoxicity (Viability; y axis on left). (F) Conformational change in the Aβ (10 μM as monomers) and HspB1 (2 μM as monomers) coassemblies was monitored by ANS fluorescence. The actual fluorescence spectra (black lines) of unassembled and oligomer samples preincubated with HspB1 were compared with the expected algebraic sums (gray lines) of the spectra for Aβ and HspB1 collected separately.