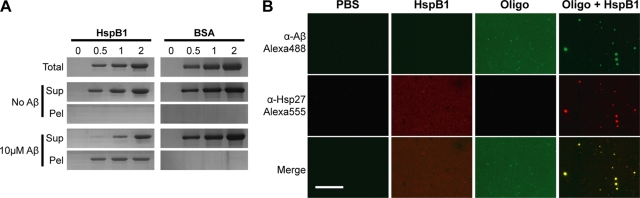
Fig. 5.
Participation of HspB1 in sequestering oligomers into aggregates. (A) Participation of HspB1 or BSA in aggregate formation was analyzed by centrifugation. Various concentrations (0.5, 1, or 2 μM as monomers) of HspB1 or BSA in the absence or presence of Aβ oligomers (10 μM as monomers) were centrifuged at 16,000 × g for 10 min. Uncentrifuged samples (Total), supernatants (Sup), and pellets (Pel) were analyzed by SDS-PAGE and stained with Coomassie blue. (B) Colocalization of Aβ and HspB1 in the aggregates. HspB1, Aβ oligomers, or oligomer-HspB1 aggregates (prepared as described for Fig. 2) were bound to polylysine-coated glass coverslips and probed with anti-Aβ (green) and anti-HspB1 (red). Bottom panels show merged images, with yellow showing colocalization. Bar, 80 μm.