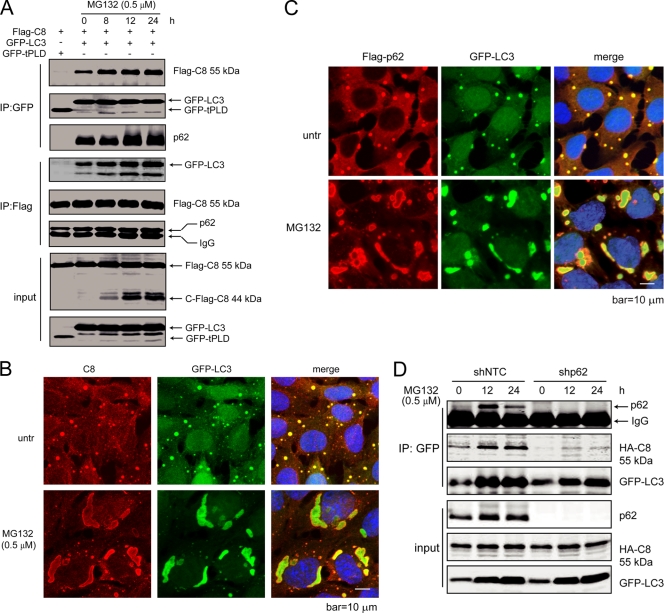
Fig. 5.
LC3 forms a complex with p62 and caspase-8, which is enhanced by proteasome inhibition. (A) Caspase-8, p62, and LC3 form a complex, which is enhanced by proteasome inhibitor treatment. HEK293 cells were transfected with plasmids encoding Flag-caspase-8, together with GFP-LC3 or a GFP control (GFP-tPLD). At 24 h posttransfection, cells with GFP-LC3 were treated with MG132 for the indicated periods of time. Cell lysates were subjected to IP with GFP or Flag antibodies and probed for the indicated proteins. (B) LC3 colocalizes with caspase-8. bax−/− bak−/− BMK cells stably expressing Flag-p62 and GFP-LC3, untreated or treated with MG132 for 18 h, were stained with an anti-caspase-8 antibody. DAPI was used to visualize the nucleus. (C) LC3 colocalizes with p62. Flag-p62 was transfected into bax−/− bak−/− BMK cells expressing GFP-LC3. Cells were left untreated or were treated with MG132 (0.5 μM) for 18 h. Immunofluorescence analysis was performed using an anti-Flag antibody. DAPI was used to visualize the nucleus. (D) p62 knockdown abolishes caspase-8 and LC3 interaction. Lentivirus encoding control shRNA (shNTC) or p62 shRNA (shp62) were used to infect bax−/− bak−/− BMK cells stably expressing HA-caspase-8 (C360S) and GFP-LC3. Cells were treated with MG132 for indicated periods of time. Cell lysates were subjected to IP with GFP and probed for indicated proteins.