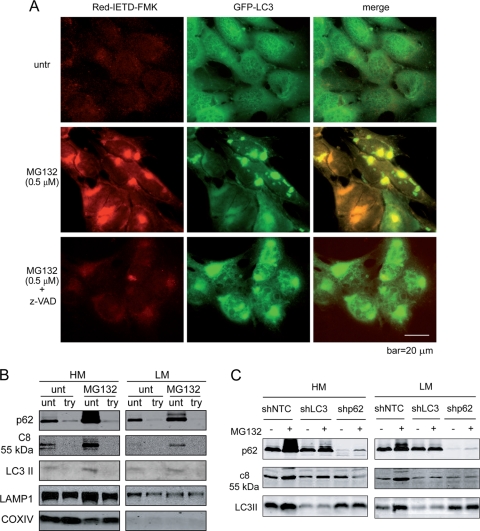
Fig. 8.
Proteasome inhibition leads to caspase-8 aggregation and activation on the cytosolic side of intracellular membranes. (A) bax−/− bak−/− BMK cells stably expressing GFP-LC3 were left untreated (one well) or were treated with MG132 (two wells). At 16 h after treatment, one well of MG132-treated cells was treated with z-VAD for 2 h. All three wells were incubated with Red-IETD-FMK for 1 h. The cells were observed under a Zeiss inverted Axiovert 200M microscope. Note that MG132 induced caspase-8 activity that colocalized with GFP-LC3. (B) bax−/− bak−/− BMK cells were left untreated or were treated with MG132 (0.5 μM) for 18 h. Cells were homogenized and left untreated or were treated with trypsin (100 μg/ml) and fractionated. Heavy-membrane (HM) and light-membrane (LM) fractions were subjected to immunoblotting for the indicated proteins. (C) MG132-induced caspase-8 membrane recruitment is dependent on both p62 and LC3. bax−/− bak−/− BMK cells expressing control shRNA (shNTC), shLC3, or shp62 were left untreated or were treated with MG132 (0.5 μM) for 18 h. The cells were fractionated. The heavy-membrane (HM) and light-membrane (LM) fractions were probed with the indicated antibodies. Note that in the LC3 and p62 knockdown cells, MG132-induced increased membrane-localized caspase-8 was diminished.