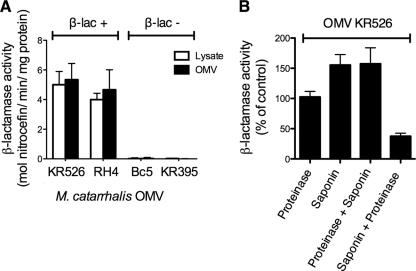
Fig. 2.
M. catarrhalis OMVs contain enzymatically active β-lactamase. (A) The β-lactamase contents of whole-cell lysates and OMVs from four different M. catarrhalis strains were analyzed. OMVs contained approximately the same β-lactamase content as whole-cell lysates. (B) The β-lactamase enzyme was found on the inside of the OMVs. The β-lactamase activity was quantified by the ability of the enzyme to hydrolyze the β-lactam nitrocefin, leading to a change in absorbance from OD380 to OD485, as determined by spectrophotometry. β-Lactamase enzyme or OMVs were treated with proteinase K (100 μg/ml) and/or saponin (0.2%), and enzyme content was subsequently determined. As a negative control, OMVs were first treated with saponin, followed by proteinase K. In panel A, the β-lactamase content was expressed as the number of moles nitrocefin hydrolyzed per minute per mg OMVs. In panel B, β-lactamase activity was defined as the percentage of enzyme activity of OMV in the absence of amoxicillin that was set to 100%. Data shown are means and SEM of at least three independent experiments.