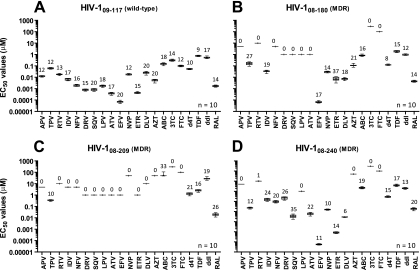
Fig. 3.
Reproducibility of drug susceptibility determination. Four p2-INT-recombinant viruses derived from treatment-naïve (A, 09-117) or treatment-experienced (B, 08-180; C, 08-209; and D, 08-240) patients were used to quantify susceptibility to all 21 antiretroviral drugs in 10 independent determinations (n = 10). The mean EC50, standard deviation, 5th to 95th percentiles, and the coefficient of variation (%) are indicated for each drug. When complete virus inhibition was not achieved using the maximum drug concentration (i.e., virus was completely resistant to a given antiretroviral drug), EC50s were not calculated, and the coefficient of variation was assigned a value of zero (0%). Mutations associated with reduction in drug susceptibility for each virus included the following: 09-117 (in PR, A71T; in RT, none; in INT, none), 08-180 (in PR, L10I, V32I, L33F, K43T, M46I, I47V, I54L, A71V, G73S, I84V, L89V, and L90M; in RT, M41L, E44D, D67N, V75M, F77L, V118I, M184V, L210F, T215Y, K219N, and N348I; in INT, L68V and E92Q); 08-209 (in PR, L10F, V32I, L33F, K43T, M46I, I54L, A71I, G73T, T74P, I84V, I85V, L89V, and L90M; in RT, A62V, D67G, K70E, V75I, F77L, K101E, V106I, Y115F, F116Y, Q151M, Y181C, M184V, and G190S; in INT, E138K, S147G, Q148R, and I203M), and 08-240 (in PR, L10F, V32I, M46I, I47V, I50V, A71I, and V82A; in RT, M41L, E44D, D67N, V118I, M184V, L210W, and T215Y; in INT, N155H). MDR, multidrug-resistant virus.