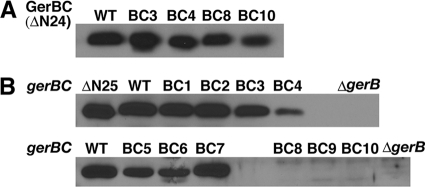
Fig. 4.
Reactivities of GerBC protein variants with anti-GerBC antiserum and levels of wild-type and variant GerBC proteins in the inner membrane of spores with the wild-type background. (A) Detection of purified wild-type and variant GerBC proteins (residues 25 to 374; 2 ng each) by Western blotting with the polyclonal anti-GerBC antiserum. (B) Detection of wild-type and variant GerBC proteins in spores' inner membrane by Western blotting with the polyclonal anti-GerBC antiserum. Inner membranes extracted from ∼1 mg dry decoated spores were run on SDS-PAGE, and purified GerBC protein (residues 25 to 374; ΔΝ24) was used as a positive control. The inner membrane fraction from spores of the gerB-null mutant (ΔgerB) (18) was used as a negative control. Note that GerBC in the inner membranes migrated slightly slower than purified GerBC, since the latter lacks the N-terminal 24 residues and cannot be diacylglycerylated.