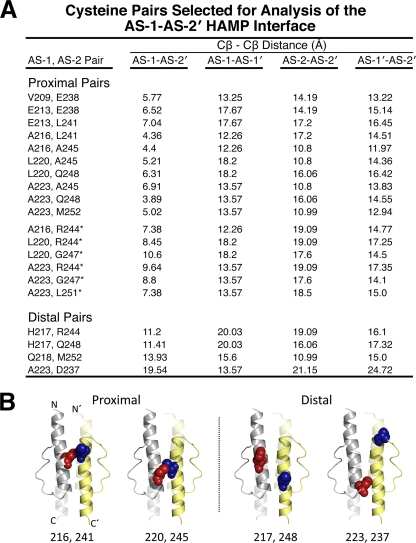
Fig. 2.
Cysteine pairs selected for analysis of the AS-1-AS-2′ HAMP interface. (A) Summary of proximal and distal di-Cys pairs and relevant β-carbon distances. Four β-carbon distances were determined for each cysteine pair on an Aer HAMP model that was generated from the coordinates of the Af1503 HAMP domain (18). Proximal pairs were selected so that AS-1-AS-2′ distances were ≤7 Å, and the three other distances (AS-1-AS-1′, AS-2-AS-2′, and AS-1′-AS-2′) were all >10 Å. Distal di-Cys control pairs had all four β-carbon distances of >10 Å. An additional set of proximal cysteine pairs were selected based on experimental data rather than on distance constraints (indicated by an asterisk) (see the text for details). (B) Examples of proximal and distal AS-1-AS-2′ pairs mapped onto the HAMP model to demonstrate their predicted intersubunit separation. AS-1 substitutions are shown as red spheres, and AS-2′ substitutions are shown as blue spheres.