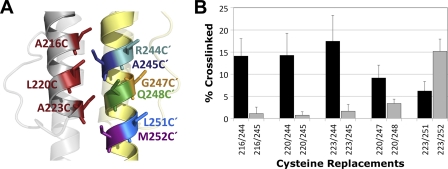
Fig. 5.
Incongruity between the HAMP model and cross-linking at residues 245C and 248C. (A) Aer HAMP model showing the location of residues 216C, 220C, and 223C in AS-1 and their predicted orientations relative to those of residues 244C′, 245C′, 247C′, 248C′, 251C′, and 252C′ in AS-2′. (B) Comparison between percent dimer formations for Aer di-Cys receptors containing R244C or A245C, G247C or Q248C, and L251C or M252C. The higher cross-linking values for R244C and G247C suggest that residues 244′ and 247′ are closer to AS-1 and/or that the orientation of these residues is different from how they are modeled in Fig. 5A. Error bars indicate the standard deviations determined from two or more independent experiments.