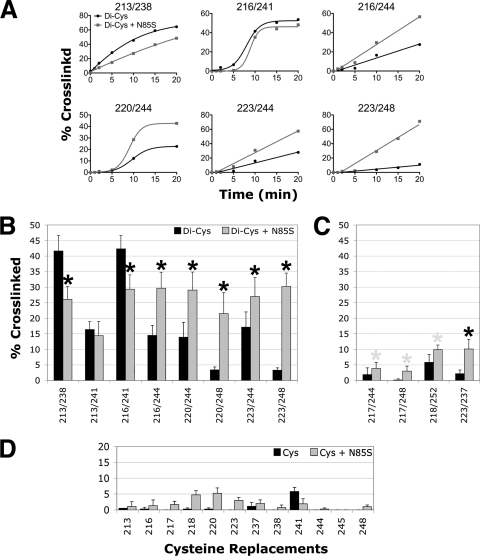
Fig. 6.
Influence of signaling state on HAMP disulfide-linked dimer formation. (A) Percent cross-linking over 20 min for representative proximal di-Cys pairs in the presence of PAS-N85S (gray lines) or the wild-type PAS sequence (black lines). (B and C) Percent dimer formation for proximal di-Cys pairs (B) and distal di-Cys pairs (C) in receptors containing PAS-N85S (gray bars) or the wild-type PAS sequence (black bars) (data from Fig. 4B and 5B). Black asterisks indicate statistically significant differences in the extents of dimer formation in the presence of N85S (P < 0.05). Some differences could reflect changes in the intersubunit disulfide formation rates between the AS-1-AS-1′ and/or AS-2-AS-2′ controls (D), and gray asterisks are used to identify these. (D) Percent dimer formation for receptors containing a single cysteine substitution in AS-1 (residues 213 to 223) or AS-2 (residues 237 to 248) in the presence (gray bars) or absence (black bars) of N85S. Error bars indicate the standard deviations determined from two or more independent experiments.