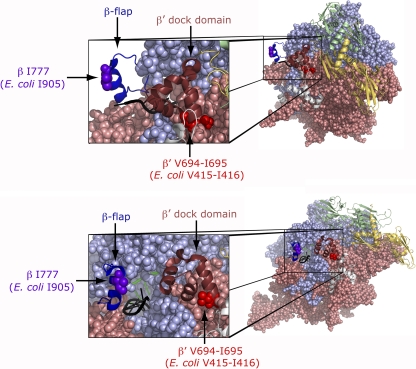
Fig. 5.
Location of the amino acid alterations, conferred by the rpoB2063 and rpoC2064 mutations in the structure of Thermus thermophilus elongating complex (PDB ID, 2O5I) (48). Two different views differing by ca. 90° are shown where the two α subunits (yellow and green, respectively) and ω (light-gray) are shown as secondary-structure cartoons, while β (light-blue) and β′ (salmon) are in space-fill. In the close-ups, the β-flap domain is shown in dark blue as a cartoon with the position altered in the rpoB2063 mutant in purple as space-fill, whereas the β′ dock domain is shown in maroon as a cartoon and at its base are the two amino acids duplicated in the rpoC2064 mutant shown in red as space-fill. The RNA emerging from the RNA exit tunnel is in black and the DNA is in green. The structures were prepared with PyMOL (13; http://www.pymol.org).