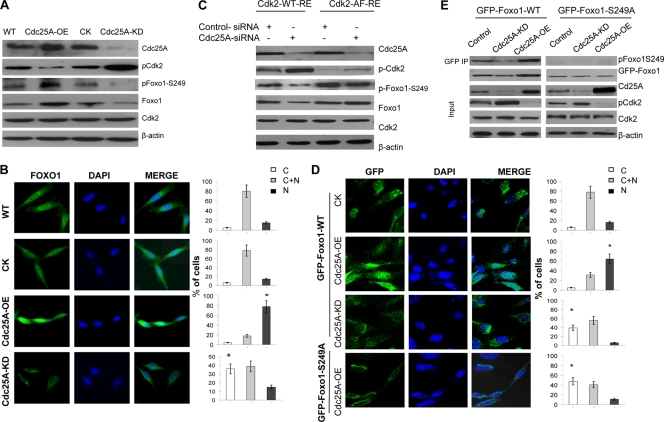
Fig. 2.
Cdc25A mediates Foxo1 phosphorylation and its cellular localization and stability by dephosphorylating Cdk2. (A) Lysates of the different cancer cells were immunoblotted with the indicated antibodies. (B) Increased or decreased nuclear Foxo1 in Cdc25A-OE (overexpression) and Cdc25A-KD (knockdown) breast cancer cells, respectively. (Left) Foxo1. (Middle) DAPI (4′,6-diamidino-2-phenylindole). (Right) Merge. Quantification of Foxo1 localization is shown on the right (bar graphs). C, cytoplasm; N, nucleus; C+N, cytoplasm and nucleus. n=200 for each cell type. *, P < 0.01 compared to the CK or WT cells. (C) The mutation Cdk2T14A Y15P interrupted the effect of Cdc25A on Foxo1. The endogenous Cdk2-depleted (by RNAi) breast cancer cells with Cdc25A-WT or Cdc25A knockdown were transfected with Cdk2-WT-RE or Cdk2-AF-RE plasmids. (D) Breast cancer cells engineered to express GFP-Foxo1 or GFP-Foxo1-S249A were assessed by localization of GFP-Foxo1 fusion proteins. (Left) GFP fluorescence. (Middle) DAPI. (Right) Merge. Quantification of GFP-Foxo1-WT or GFP-Foxo1-S329A (bottom) is shown on the right (bar graphs). n=200 for each cell type. *, P < 0.01 compared to the control cells. (E) The indicated breast cancer cells engineered to express exogenous GFP-Foxo1 were harvested, and the lysates were immunoprecipitated with GFP antibody. Similar results were observed using MDA-MB-231 and MDA-MB-453 breast cancer cells versus their derivatives.