Fig. 7.
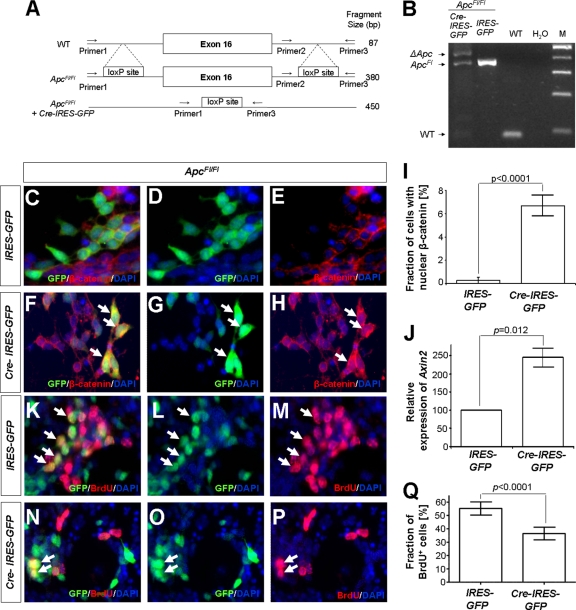
Acute loss of Apc resulted in significantly reduced proliferation of primary cultured cerebellar granule neuron precursors. The scheme shows the Apc wild-type (WT) allele, as well as the Apc allele with loxP sites before and after exposition to Cre recombinase (A). PCR of isolated DNA from ApcFl/Fl granule neuron precursors that were cultured at P7 and transduced with either IRES-GFP or Cre-IRES-GFP virus was carried out using all three indicated primers. The gel shows successful recombination resulting in a fragment of 450 bp after treatment with Cre-IRES-GFP virus (B). Primers flanking the right loxP insertion site produced a fragment of 380 bp in both Cre-IRES-GFP- and IRES-GFP-treated cultures from ApcFl/Fl mice but not in WT animals, where only 87 bp were amplified (B). Note that the DNA fragment between primers 1 and 3 was not amplified in unrecombined cells due to its large size. Granule neuron precursor cells were cultured from ApcFl/Fl mice at P7 and transduced with either Cre-IRES-GFP or IRES-GFP virus. Staining with antibodies against β-catenin featured nuclear accumulation in only 0.35% of cells transduced with IRES-GFP (C to E and I) but in 6.5% of cultured cells treated with Cre-IRES-GFP (G and H, arrows; I; P < 0.0001, Fisher exact test). Quantitative RT-PCR revealed a significant increase in the transcriptional activity of the Wnt target gene Axin2 in cultures from ApcFl/Fl mice treated with Cre-IRES-GFP virus (J) (P = 0.012, Student's t test). Statistical analysis of subsequent immunocytochemical stainings using antibodies against BrdU revealed significantly fewer proliferating cells under conditions with Cre-IRES-GFP virus treatment (N to Q) than under control conditions (K to M and Q; P = 0.015, Student's t test). Values in panel Q are shown as means ± SEM from 3 independent experiments.