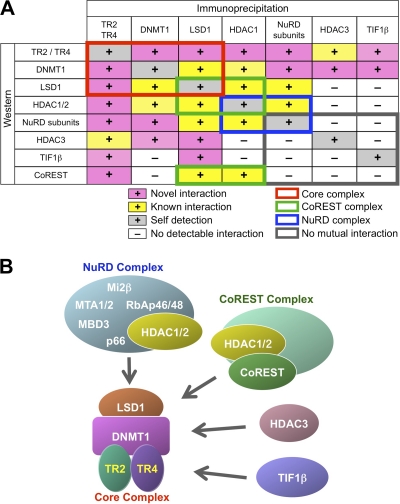
Fig. 8.
Multiple TR2/TR4 repressor complexes in adult erythroid (MEL) cells. (A) Summary of coimmunoprecipitation experiments using normal MEL cells. Novel interactions identified in the present study are shown as plus signs on a pink background, whereas previously reported interactions are shown on a yellow background. Detection of a protein immunoprecipitated with an antibody against it is indicated on a gray background as a positive control (self-detection). Known protein interactions between components of the NuRD and CoREST repressor complexes are shown in blue and green rectangles, respectively. TR2, TR4, DNMT1, and LSD1 interactions are indicated by a red rectangle and comprise a core complex (shown in panel B). These four core constituents commonly interact with additional proteins, such as HDAC1/2, NuRD components (MTA1/2, Mi2β, or RbAp46/48), HDAC3, TIF1β, or CoREST, thus forming larger complexes. However, no mutual interactions between NuRD components (except HDAC1/2), HDAC3, TIF1β or CoREST were detected by coimmunoprecipitation (indicated by the gray rectangle), suggesting that NuRD, HDAC3, TIF1β, and the CoREST complex bind to the core complex in a mutually exclusive manner, thus forming at least four distinct larger complexes that share the common (TR2+TR4+DNMT1+LSD1) core. (B) Structural models for multiple TR2/TR4 repressor complexes. Based on the results of the coimmunoprecipitation assays, we propose a structural model that contains multiple and diverse activities as TR2/TR4 repressor complexes. TR2, TR4, DNMT1, and LSD1 comprise the core negative regulatory silencing complex, to which the NuRD or CoREST complex, HDAC3, or TIF1β binds in a mutually exclusive manner to form at least four distinct larger complexes that share the core complex.