Fig. 2.
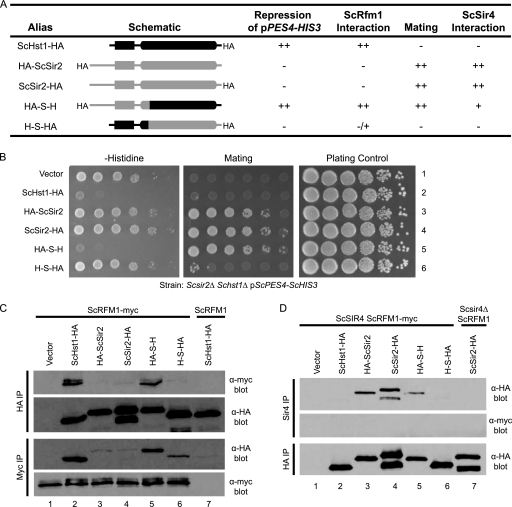
Distinct portions of ScSir2 and ScHst1 are required for specificity. (A) Summary of chimeric proteins and their properties. (B) Hst1-mediated repression and Sir2-mediated silencing were assessed using a pPES4–HIS3 reporter and a mating assay. A Scsir2Δ Schst1Δ mutant strain (LRY2083) was transformed with an empty vector (pRS416) or plasmids expressing the constructs shown in panel A (Table 2). To assess ScHst1-mediated repression (left side), yeast cells were spotted onto medium lacking histidine and uracil (for plasmid maintenance) in 10-fold serial dilutions. To assess ScSir2-mediated silencing (middle), the same dilutions were mixed with a tester strain of the opposite mating type (LRY1022) and spotted onto minimal medium to select for prototrophic diploid cells. As a plating control (right side), yeast cells were spotted onto rich medium. (C) The association of chimeric proteins with ScRfm1-myc was examined by co-IP. HA-tagged protein or ScRfm1-myc was immunoprecipitated from an Scsir2Δ Schst1Δ ScRFM1–myc mutant strain (LRY2507) transformed with plasmids expressing the constructs in panel A or an Scsir2Δ Schst1Δ ScRFM1 mutant strain (LRY2083) transformed with ScHST1-HA (lane 7). The precipitated material was examined by immunoblotting with mouse anti-HA or anti-myc antibody. Two major bands were observed for C-terminally HA-tagged ScSir2 but not the N-terminally tagged protein. The larger band (∼70 kDa) is close to the predicted size of full-length ScSir2-HA. Others have also observed multiple bands for C-terminally tagged ScSir2 constructs (14a, 34) and have postulated that the smaller bands are degradation products. However, as multiple bands are not seen for N-terminally tagged ScSir2, the smaller band could result from internal translation initiation. (D) The association of chimeric proteins with ScSir4 was examined by co-IP. ScSir4 was immunoprecipitated from the same strains used in panel C or an Scsir2Δ Schst1Δ Scsir4Δ mutant strain (LRY2590) transformed with ScSIR2-HA (lane 7).