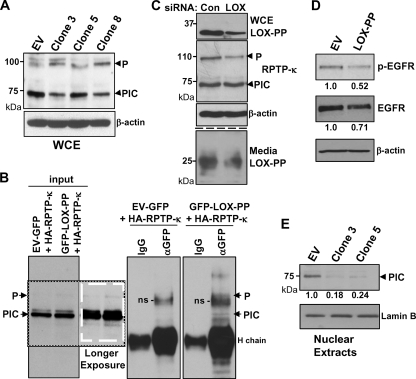
Fig. 4.
LOX-PP expression induces changes in the levels and distribution of RPTP-κ isoforms in H1299 cells. (A) Control (EV) or LOX-PP-expressing clones 3, 5, and 8 were incubated in the presence of Dox for 48 h. Whole-cell extracts (WCE) were prepared in RIPA buffer and subjected to immunoblotting for RPTP-κ and β-actin, as a loading control. (B) H1299 cells were transfected with an HA-tagged RPTP-κ expression plasmid (3 μg) in combination with 3 μg of vector expressing either GFP (control) or GFP-LOX-PP. After 48 h, whole-cell lysates were prepared. Following removal of samples for input lanes (17%), the lysates were subjected to immunoprecipitation with an antibody directed against either GFP (αGFP) or control IgG (IgG) and immunoprecipitated proteins analyzed by Western blotting for RPTP-κ. Please note that the lanes are all from the same gel/blot but cut to align in the correct order. A darker exposure of a section of the input lanes is shown, in a white box, to better visualize the P subunit. Positions of the precipitated P subunit and PIC isoform are as indicated. H chain, immunoglobulin heavy chain; ns, nonspecific band. (C) H1299 cells were incubated with either 20 nM control siRNA (Con) or 10 nM (each) LOX siRNAs 1 and 2 (LOX) using Lipofectamine RNAiMax. After 48 h, medium was collected and whole-cell extracts prepared. Intracellular expression of LOX-PP, RPTP-κ, and β-actin (upper panels) and extracellular LOX-PP levels (lower panels) were monitored as described in the Fig. 3 legend except that the Novus anti-human LOX-PP antibody was used. (D) A549 cells were transiently transfected with EV DNA or LOX-PP expression vector. After 24 h, cultures were incubated overnight in medium containing 0.5% FBS and stimulated with 100 ng/ml EGF for 15 min, and whole-cell extracts subjected to Western blotting for p-EGFR, EGFR, and β-actin. The levels of p-EGFR and EGFR in this and one independent replicate experiment were quantified. The average values normalized to the results for the β-actin loading control from the two experiments are given below, relative to the results for control EV DNA (set to 1.0). (E) Control (EV) or LOX-PP clones 3 and 5 were induced with Dox for 48 h. The nuclear fraction was prepared and subjected to immunoblotting for RPTP-κ and for lamin B as a loading control. The results from three independent experiments were subjected to densitometry and normalized to the result for the lamin B loading control, and the mean values relative to the results for control EV cells (set to 1.0) are given.