Abstract
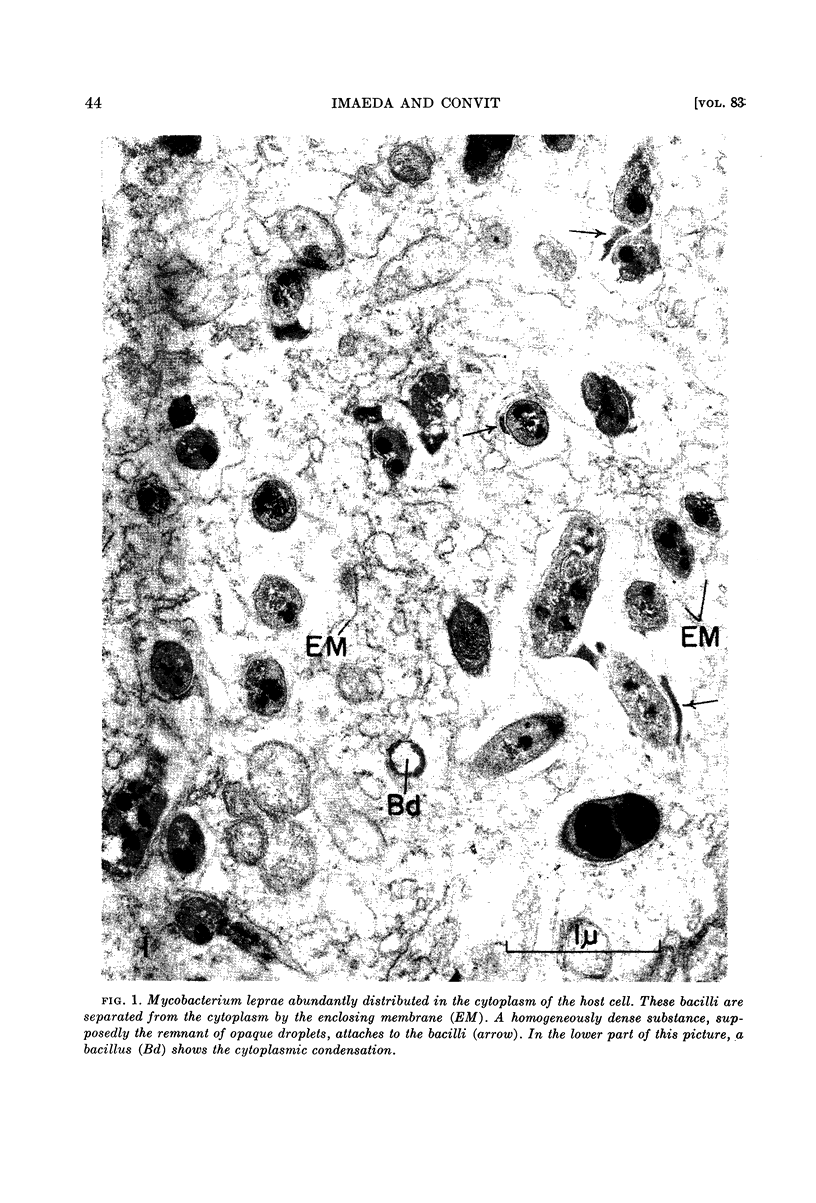
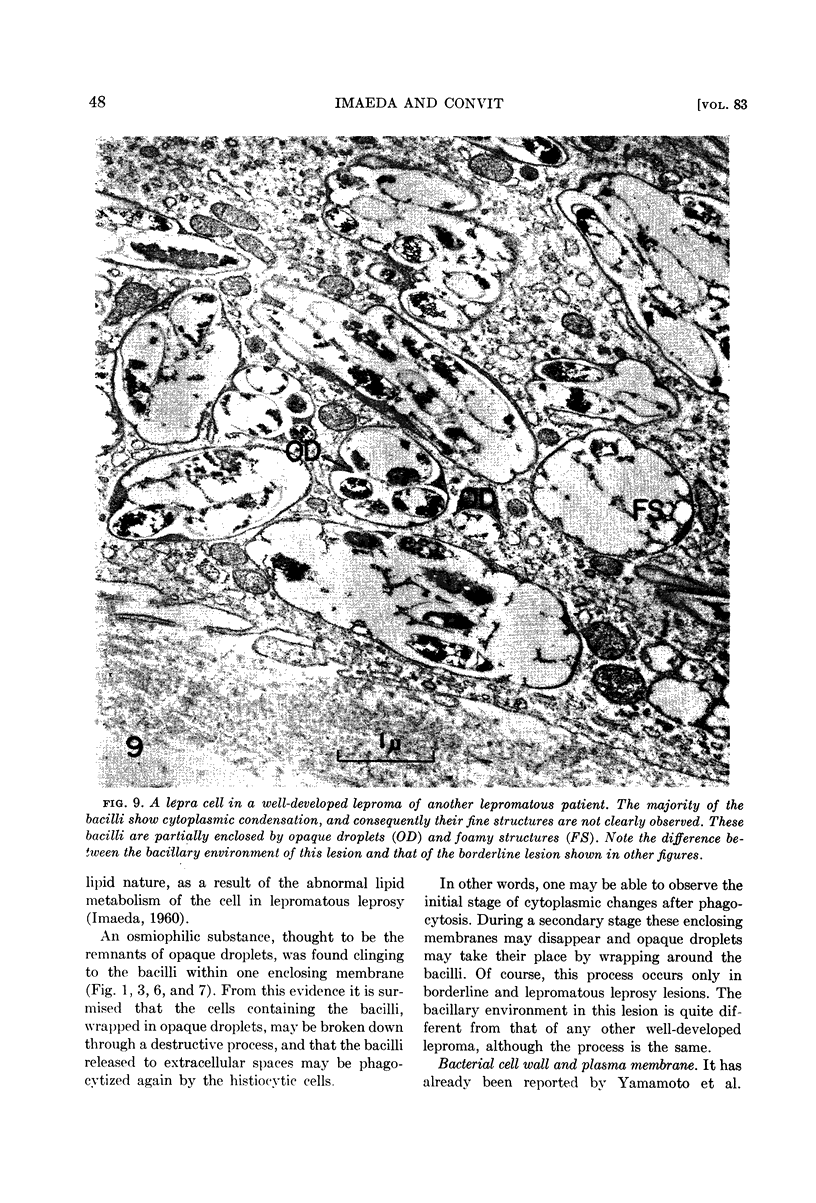
Imaeda, Tamotsu (Instituto Venezolano de Investigaciones Cientificas, Caracas, Venezuela) and Jacinto Convit. Electron microscope study of Mycobacterium leprae and its environment in a vesicular leprous lesion. J. Bacteriol. 83:43–52. 1962.—Biopsied specimens of a borderline leprosy lesion were observed with the electron microscope. In this lesion, the majority of Mycobacterium leprae were laden with cytoplasmic components. The bacilli were separated from the cytoplasm of host cells by an enclosing membrane, thus differing from the environment of well-developed lepra cells in lepromatous lesions.
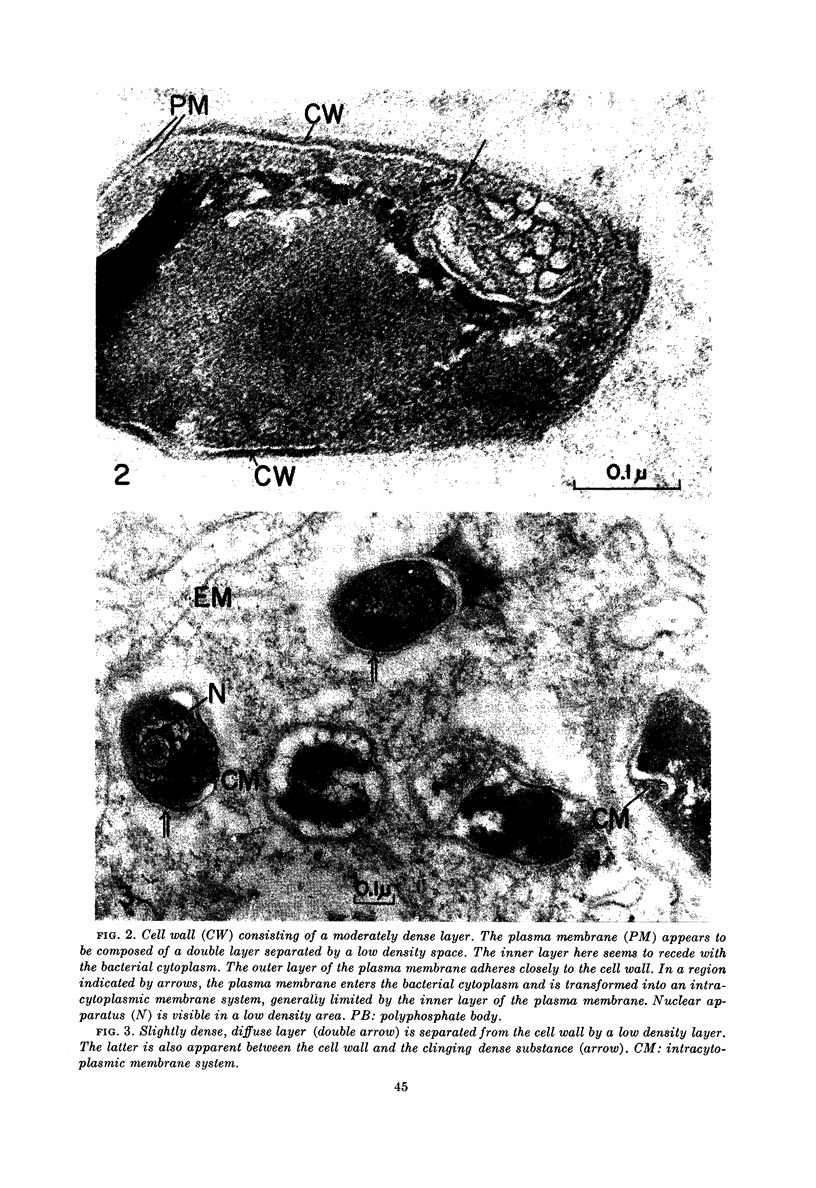
The cell wall is composed of a moderately dense layer. A diffuse layer is discernible outside the cell wall, separated from it by a low density space. It is suggested that the cell wall is further coated by a low density layer, although the nature of the outermost diffuse layer has not yet been determined.
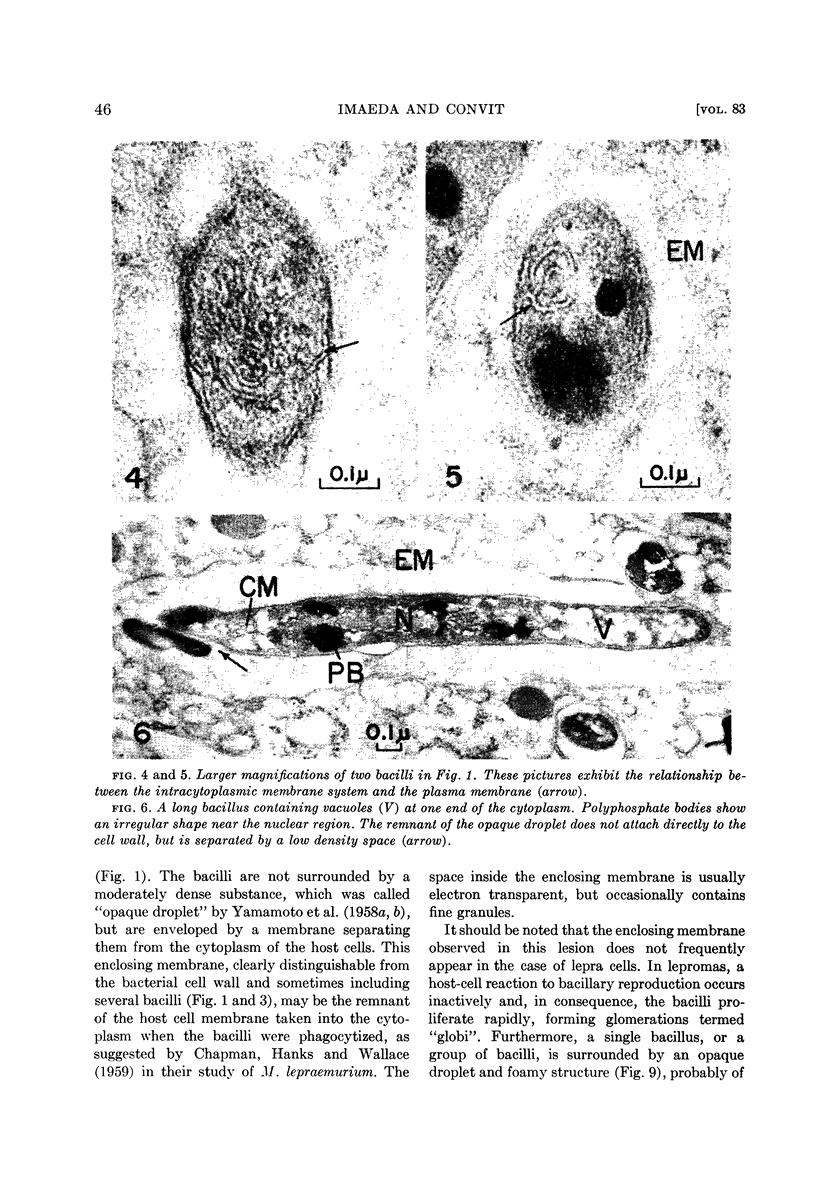
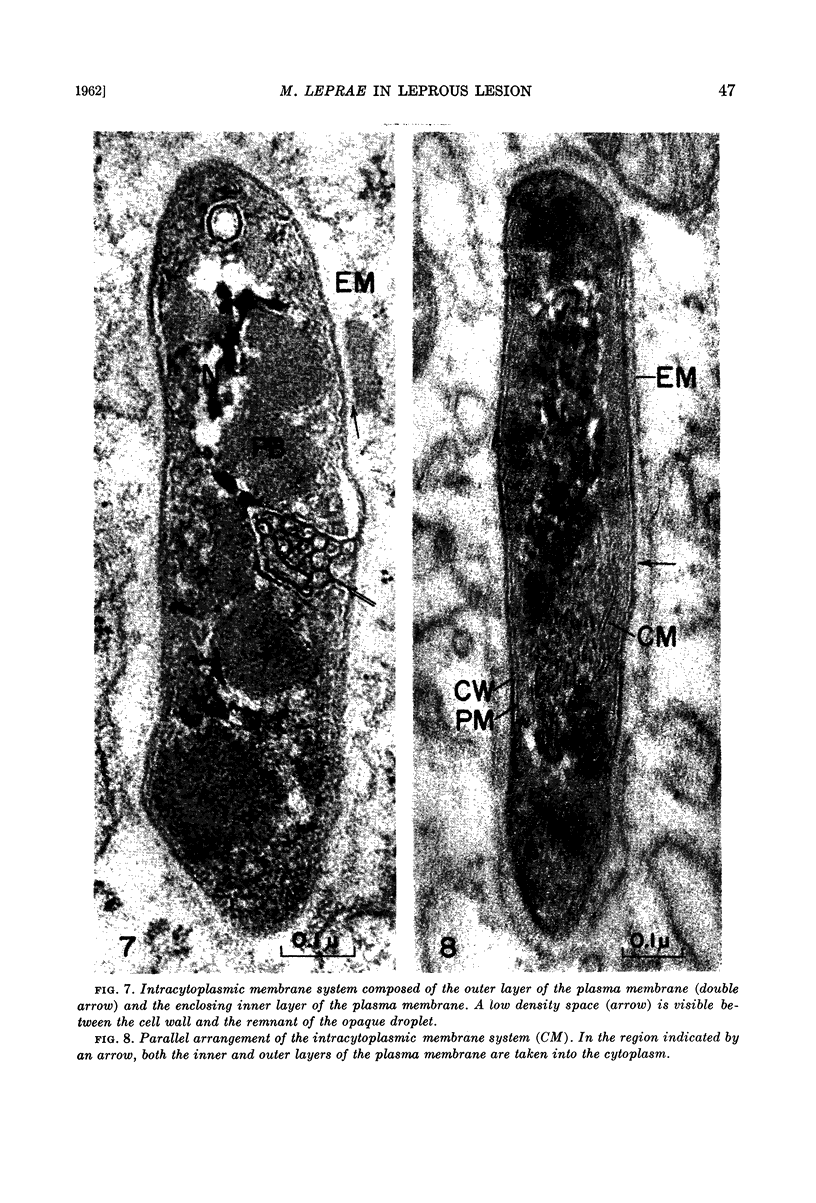
The plasma membrane consists of a double layer, i.e., dense inner and outer layers separated by a low density space. The outer layer is closely adjacent to the cell wall. In the region where the outer layer of the plasma membrane enters the cytoplasm and is transformed into a complex membranous structure, the inner layer encloses this membranous configuration. Together they form the intracytoplasmic membrane system.
In the bacterial cytoplasm, moderately dense, presumably polyphosphate bodies are apparent. As neither these bodies nor the intracytoplasmic membrane system are visible in the degenerating bacilli, it seems probable that these two components represent indicators of the state of bacillary activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRIEGER E. M., GLAUERT A. M., ALLEN J. M. ytoplasmic structure in Mycobacterium leprae. Exp Cell Res. 1959 Oct;18:418–421. doi: 10.1016/0014-4827(59)90032-1. [DOI] [PubMed] [Google Scholar]
- CHAPMAN G. B., HANKS J. H., WALLACE J. H. An electron microscope study of the disposition and fine structure of Mycobacterium lepraemurium in mouse spleen. J Bacteriol. 1959 Feb;77(2):205–211. doi: 10.1128/jb.77.2.205-211.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATTERJEE K. R., DAS GUPTA N. N., DE M. L. Electron microscopic observations on the morphology of Mycobacterium leprae. Exp Cell Res. 1959 Nov;18:521–527. doi: 10.1016/0014-4827(59)90317-9. [DOI] [PubMed] [Google Scholar]
- DREWS G. [Electron microscopic studies on Mycobacterium phlei. (Structure and formation of metachromatic granula)]. Arch Mikrobiol. 1960;35:53–62. [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUSHI K. Electron microscopic studies of tubercle bacilli. V. Studies on fixation in ultrathin sectioning. Sci Rep Res Inst Tohoku Univ Med. 1959 Dec;9:1–17. [PubMed] [Google Scholar]
- GLAUERT A. M., BRIEGER E. M., ALLEN J. M. The fine structure of vegetative cells of Bacillus subtilis. Exp Cell Res. 1961 Jan;22:73–85. doi: 10.1016/0014-4827(61)90087-8. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. The fine structure of Streptomyces coelicolor. I. The cytoplasmic membrane system. J Biophys Biochem Cytol. 1960 Jun;7:479–488. doi: 10.1083/jcb.7.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDERSON H. J., MUDD S., TAKEYA K. Electron-scattering granules and reducing sites in mycobacteria. J Bacteriol. 1956 Dec;72(6):767–783. doi: 10.1128/jb.72.6.767-783.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMAEDA T. Electron microscopic analysis of the components of lepra cells. Int J Lepr. 1960 Jan-Mar;28:22–37. [PubMed] [Google Scholar]
- KOIKE M., TAKEYA K. Fine structures of intracytoplasmic organelles of mycobacteria. J Biophys Biochem Cytol. 1961 Mar;9:597–608. doi: 10.1083/jcb.9.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALFATTI M. G. Investigaciones sobre la morfologäa del Mycobacterium leprae a través de la óptica electrónica. Sem Med. 1951 Jul 19;99(3):109–118. [PubMed] [Google Scholar]
- MUDD S., YOSHIDA A., KOIKE M. Polyphosphate as accumulator of phosphorus and energy. J Bacteriol. 1958 Feb;75(2):224–235. doi: 10.1128/jb.75.2.224-235.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIURA M. The electron microscopic basis of the pathology of leprosy. Int J Lepr. 1960 Oct-Dec;28:357–400. [PubMed] [Google Scholar]
- ROBERTSON J. D. The ultrastructure of cell membranes and their derivatives. Biochem Soc Symp. 1959;16:3–43. [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- SHINOHARA C., FUKUSHI K., SUZUKI J. Mitochondria-like structures in ultrathin sections of Mycobacterium avium. J Bacteriol. 1957 Sep;74(3):413–415. doi: 10.1128/jb.74.3.413-415.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEYA K., MORI R., KOIKE M., TODA T. Paired fibrous structure in mycobacteria. Biochim Biophys Acta. 1958 Oct;30(1):197–198. doi: 10.1016/0006-3002(58)90265-8. [DOI] [PubMed] [Google Scholar]
- VAN ITERSON W., ROBINOW C. F. Observations with the electron microscope on the fine structure of the nuclei of two spherical bacteria. J Biophys Biochem Cytol. 1961 Jan;9:171–181. doi: 10.1083/jcb.9.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON W. Some features of a remarkable organelle in Bacillus subtilis. J Biophys Biochem Cytol. 1961 Jan;9:183–192. doi: 10.1083/jcb.9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C., BERGSTROM L. The chemical nature of the cytoplasmic membrane and cell wall of Bacillus megaterium, strain M. Biochim Biophys Acta. 1958 Nov;30(2):340–351. doi: 10.1016/0006-3002(58)90059-3. [DOI] [PubMed] [Google Scholar]
- ZAPF K. Vergleichende Untersuchungen zur Morphologie und Zytologie des Mycobakterium tuberculosis (BCG). II. Licht-und elektronenmikroskopische Befunde zum Kernproblem. Zentralbl Bakteriol Orig. 1959 Feb;174(3-4):253–263. [PubMed] [Google Scholar]